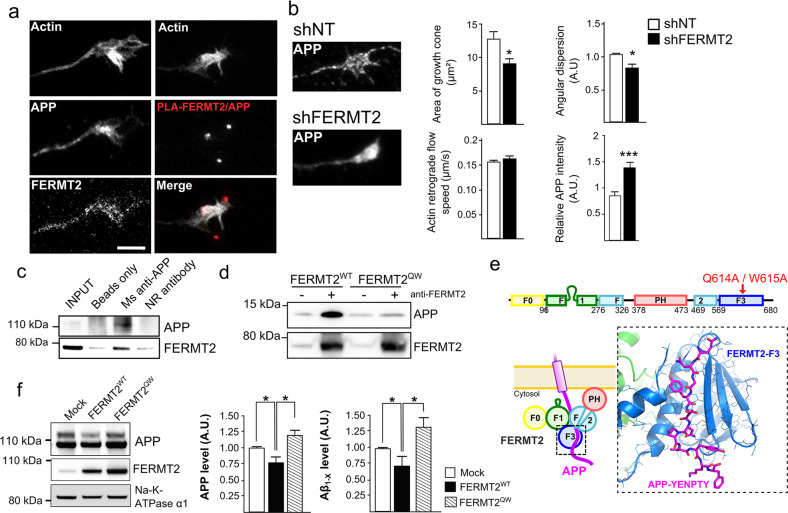
Fig. 4. FERMT2 directly interacts with APP.
a Immunofluorescence images showing the presence of APP and FERMT2 within the axonal growth cone stained with SiR-Actin. The right panel shows the presence of PLA-FERMT2/APP puncta within the axonal growth cone. b Impact of lentiviral transduction of nontargeting shRNA (shNT) or shRNA against FERMT2 (shFERMT2) on growth cone area, angular dispersion and speed of the actin-retrograde flow, and APP immunostaining. c Co-IP between endogenous APP and FERMT2 from membrane extracts of hippocampal PNC. Protein extracts were incubated with beads only, a mouse (Ms) antibody against APP (4G8), or a nonrelevant (NR) antibody. d APP pull-down experiment with wild-type (WT) or mutated (QW) FERMT2. Protein extracts from HeLa cells overexpressing FERMT2WT or FERMTQW were incubated with recombinant APP C-terminal fragment (C100). e The domain organization of FERMT2 protein (upper panel). Q614A/W615A (QW) mutation was reported to abolish the interaction of F3 domain of FERMT2 with the NxTY motif. The structural model of the FERMT2-APP complex (lower panel) was built by homology using the crystal structure of the FERMT2-Integrin-β3-tail complex [32]. f The impact of FERMT2 on APP metabolism in HEK293-APP695WT cells is reverted with the overexpression of FERMT2QW compared to FERMT2WT. Scale bar = 5 µm. Mann–Whitney test; *p < 0.05.