Figure 6.
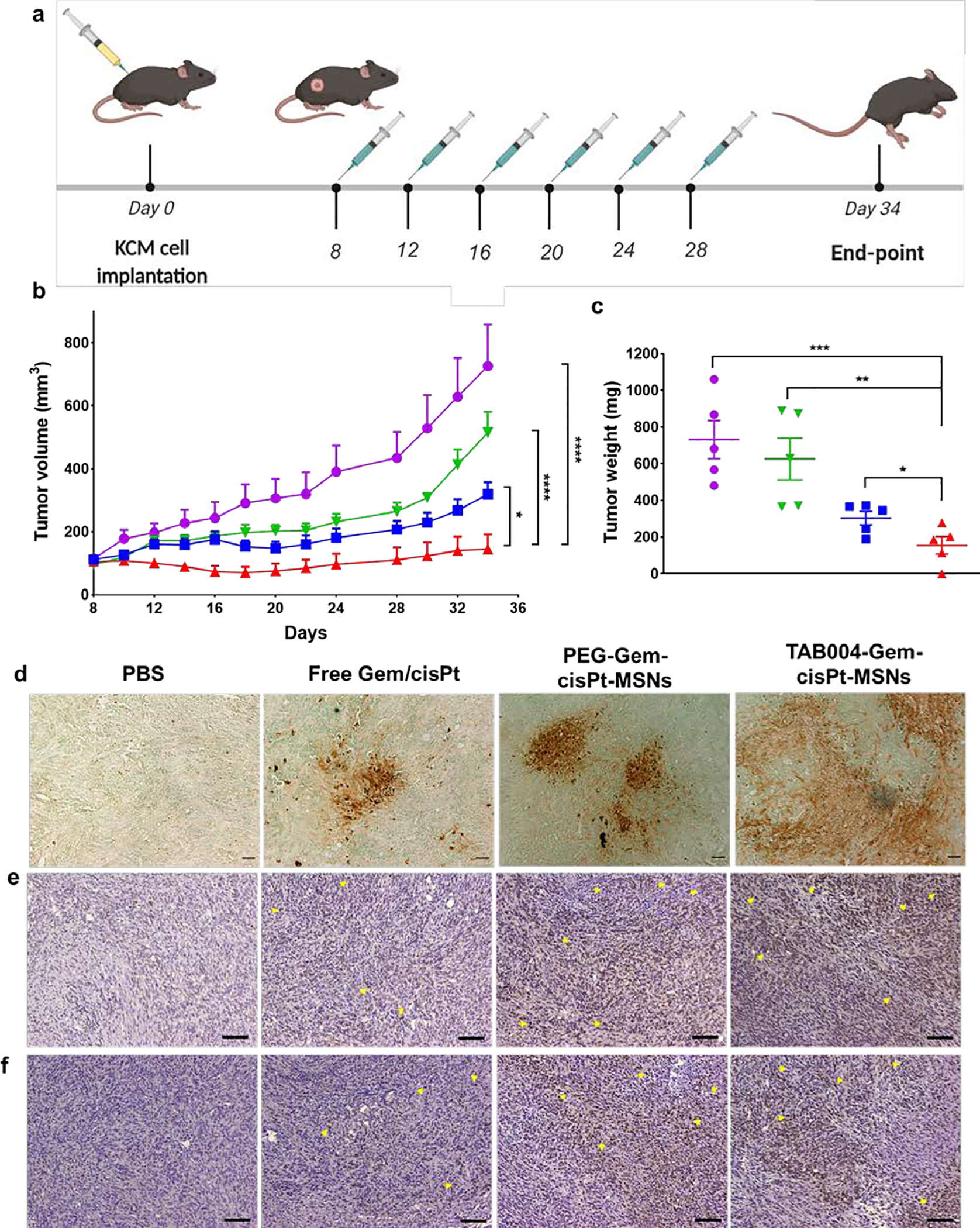
In vivo therapeutic efficacy of TAB004-Gem-cisPt-MSNs in syngeneic KCM mice. (a) Schematic representation of the treatment regimen: mice were injected with MSN materials or free drugs 6 times with a 4-day interval between each injection. (b) Tumor volume was measured throughout the study in the following treatment groups: PBS (purple), Free Gem/cisPt (green), PEG-Gem-cisPt-MSNs (blue), and TAB004-Gem-cisPt-MSNs (red) (n=5 mice per group). Two-way ANOVA was performed between the groups and time points, to determine whether outcomes were statistically different. (c) Tumor weights measured at the endpoint of the efficacy studies. T-test was performed to determine whether the weights between groups were statistically different. Statistics: ****p≤0.0001, ***p≤0.001, ** p≤0.01, * p≤0.05 and not significant (ns) p>0.05. Ex vivo analysis of tumor sections assessed to show the number of apoptotic cells (d), γH2AX positive cells (e), phosphorylated Chk1 (Chk1P) positive cells (f) in tumors post treatment. Scale bar = 100 μm (d) and 200 μm (e and f).
