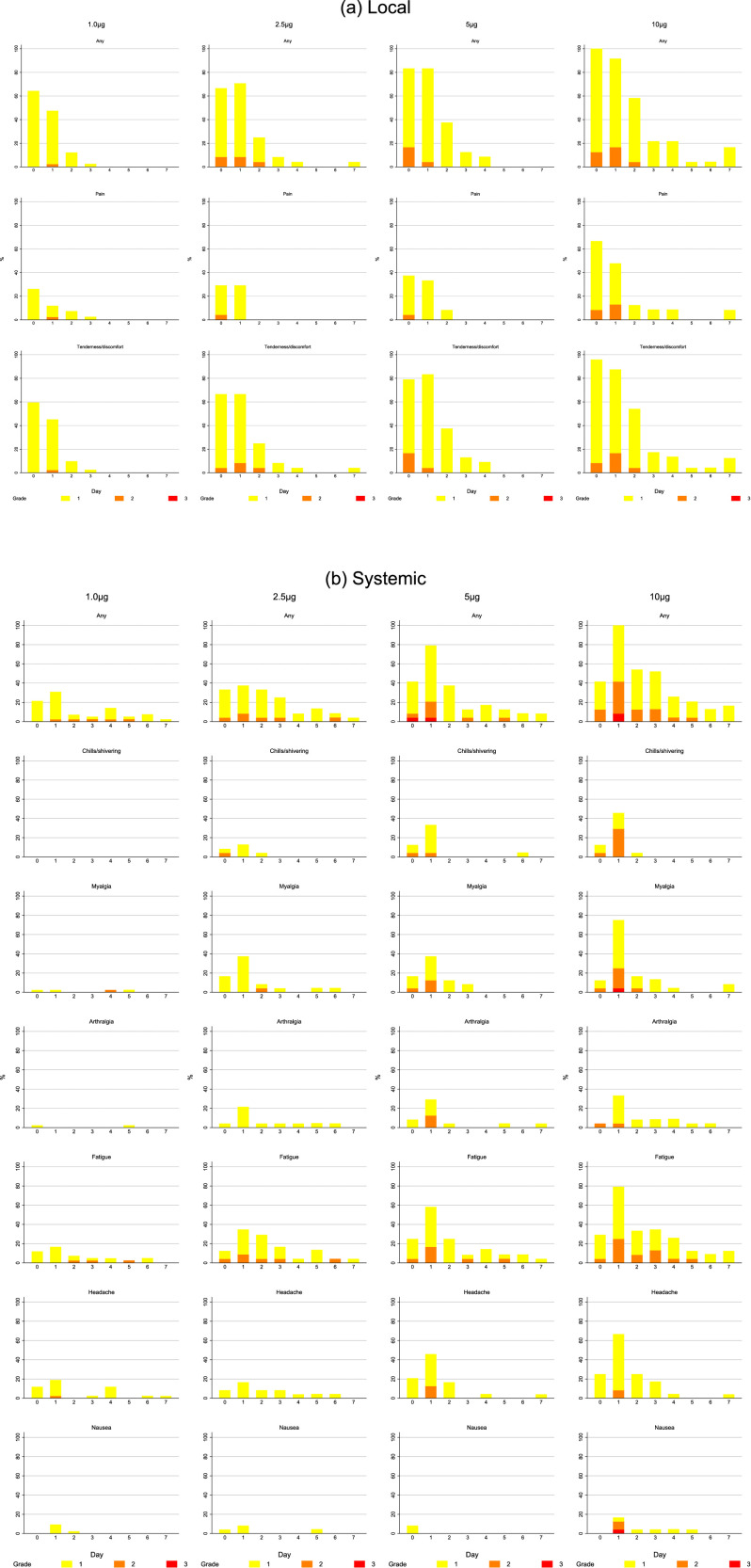
Figure 2.
A. Solicited local injection site reactions that started within 7 days of administration of the vaccine with a frequency of at least 10%. Reactions are shown after the first injection in those who received, in columns from left to right 1.0μg, 2.5μg, 5.0μg, and 10.0μg. The upper row shows reports of any solicited local injection site reaction, the middle row pain at the injection site and the lower row tenderness at the injection site on the day of vaccination and for 7 days afterwards. Grade of adverse event is represented by colour on the bar chart as grade 1 (mild) in yellow, grade 2 (moderate) in orange and grade 3 (severe) in red. Figure 2B. Solicited systemic reactions that started within 7 days of administration of the vaccine with a frequency of at least 10%. Reactions are shown after the first injection in those who received in columns from left to right, 1.0μg, 2.5μg, 5.0μg, and 10.0μg. Rows show from the top any solicited systemic reaction, chills/shivering, myalgia, arthralgia, fatigue, headache and nausea. Grade of adverse event is represented by colour on the bar chart as grade 1 (mild) in yellow, grade 2 (moderate) in orange and grade 3 (severe) in red.