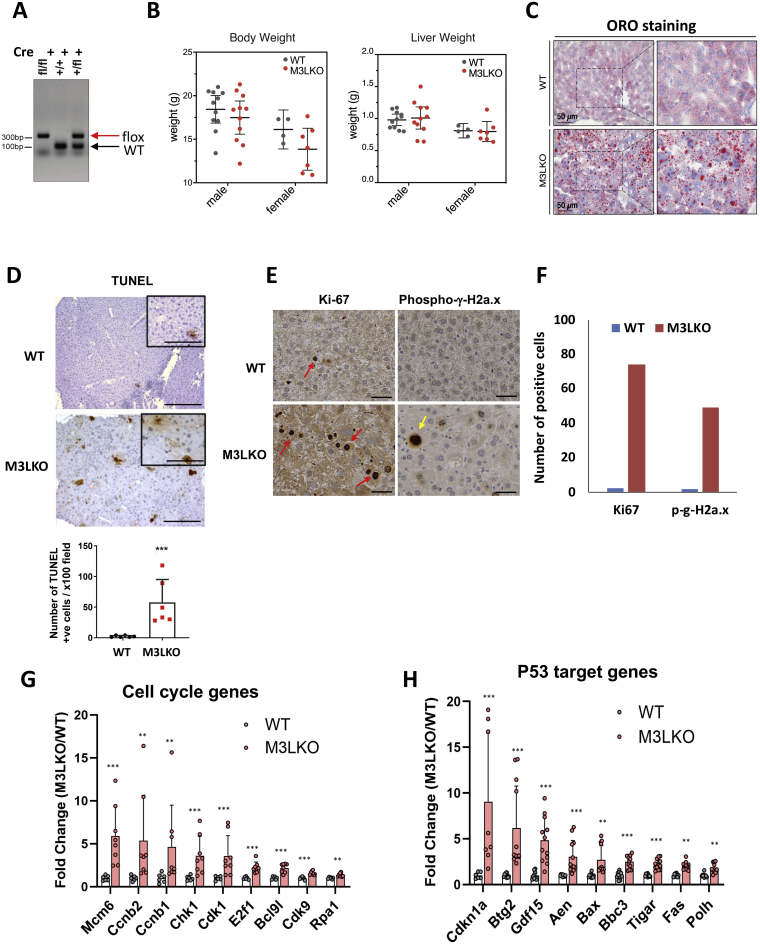
Supplemental Figure S1.
Generation and characterization of liver-specific METTL3 knockout (M3LKO) mice. M3LKO (Mettl3fl/fl; Alb-Cre) mice in C57BL/6 background were generated by crossing Mettl3fl/fl mice with Alb-Cre mice to delete Mettl3 specifically in parenchymal cells at the neonatal stage. A: Confirmation of genotypes of the wild-type (WT) (Mettl3+/+), heterozygous (Mettl3fl/+), and homozygous (Mettl3fl/fl) M3LKO mice by tail DNA PCR. B: Body and liver weights of 5-week–old WT and homozygous M3LKO mice. C: Microsteatosis in M3LKO livers confirmed by Oil-Red-O staining of frozen liver sections of 5-week–old mice. D: A terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay demonstrating hepatocyte apoptosis in 10-week–old M3LKO male mice. Representiative pictures are shown. Quantification of TUNEL-positive cells were counted using ImageJ software version 1.52a (NIH, Bethesda, MD; http://imagej.nih.gov/ij). E: Representative pictures of Ki-67 and phopho-g-H2a.x immunohistochemistry and relative numbers of Ki-67–positive (red arrows) and phopho-g-H2a.x (yellow arrows) hepatocytes in 10-week–old male WT and M3LKO mice. F: Numbers of Ki-67–positive and phospho-g-H2a.x+ hepatocytes are presented. G and H: RNA sequencing data demonstrating dysregulation of P53-target genes and cell cycle regulatory genes, respectively, in 5-week–old M3LKO livers. n = 3 per genotype. ∗∗P < 0.01, ∗∗∗P < 0.001 (Wald test or t-test). Scale bars: 50 μm (C); 100 μm (D, inset; E); 200 μm (D).