Scheme 135. Synthetic Strategies Toward the Preparation of peri-Xanthenoxanthene Imides.
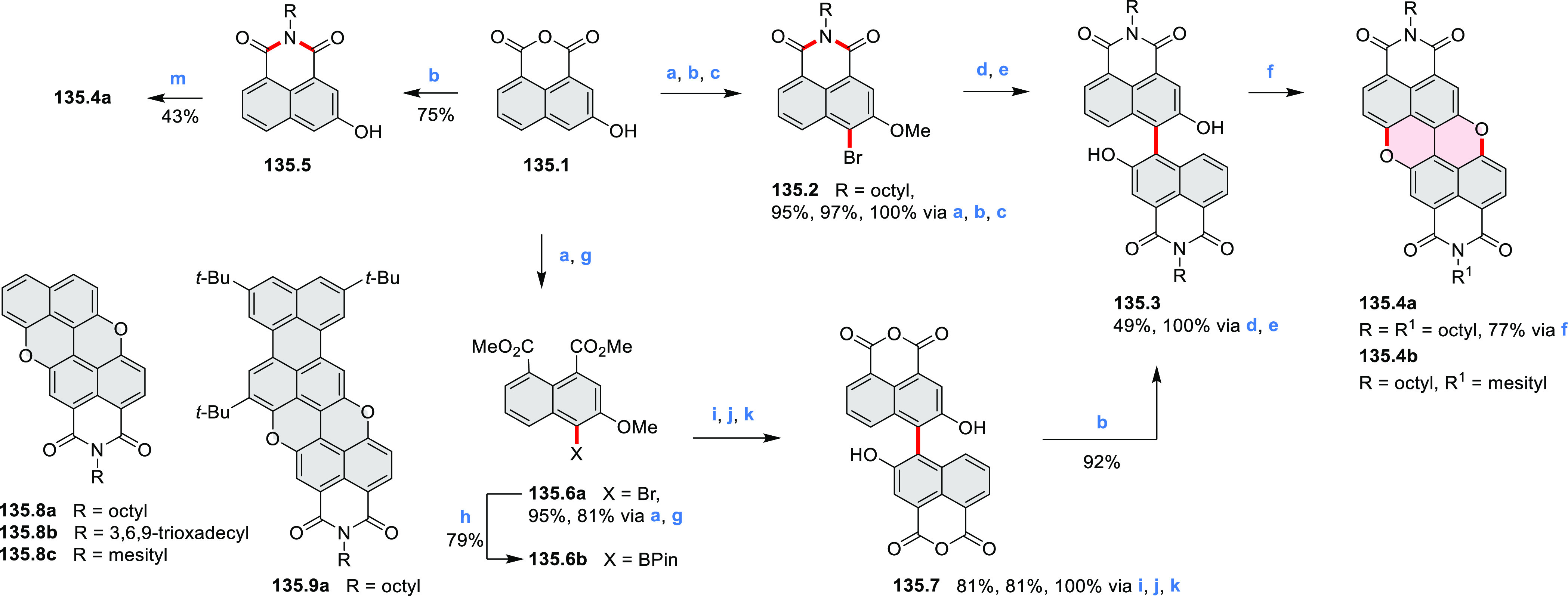
Reagents and conditions: (a)271,272 Br2, dioxane, reflux, 2.5 h; (b) i-Pr2NEt, n-octylamine, dioxane, reflux, 16–24 h; (c) K2CO3, CH3I, MeCN, reflux, 4 h; (d) Cs2CO3, B2Pin2, Pd(dba)2, SPhos, dioxane, reflux, 18 h; (e) BBr3, DCM, 0 °C to rt, 16 h; (f) pivalic acid, CuI, DMSO, 120 °C, 5 h; (g) DBU, MeI, MeOH, reflux, 18 h; (h) B2Pin2, KOAc, Pd(PPh3)2Cl2, 1,4-dioxane, reflux, 16 h; (i) 135.6a, 135.6b, K3PO4, Pd(dba)2, SPhos, 1,4-dioxane, H2O, reflux; (j) (1) KOH, i-PrOH, reflux, 13 h, (2) HCl(aq.), AcOH, reflux, 24 h; (k) HBr(aq.), AcOH, 126 °C, 24 h; (m) CuCl, DMSO, 120 °C, 24 h, air.
