Scheme 166. Pyrene-Fused Imidazolium Cations and Their NHC Complexes.
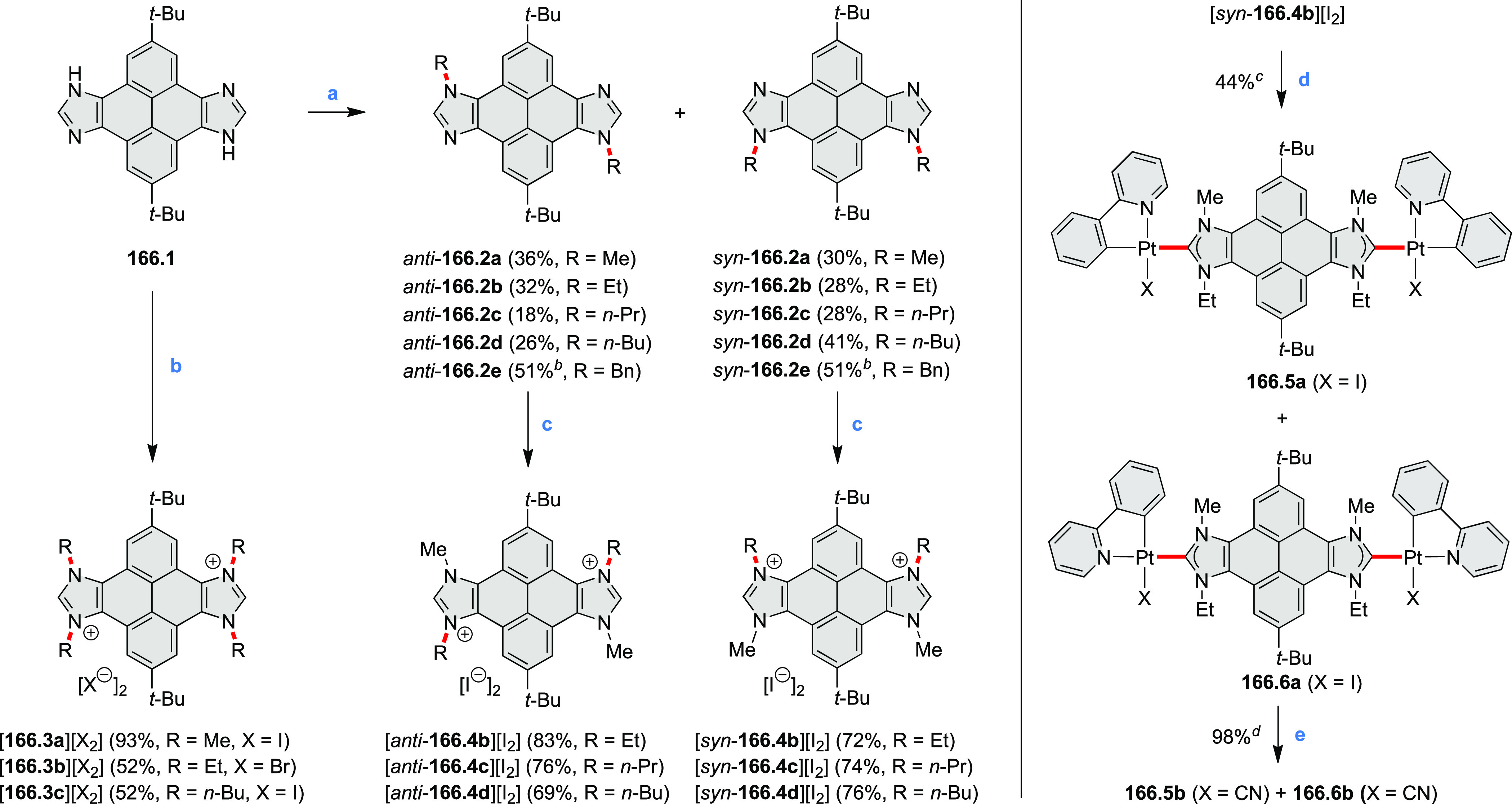
Reagents and conditions: (a)335 (1) NaOH, DMSO, rt, 2 h, (2) RBr or RI (2 equiv), rt for 30 min, then 37 °C, overnight; (b) (1) NaOH, DMSO, rt, 2 h, (2) RBr or RI (excess), rt for 30 min, then 37 °C, overnight; (c) MeI (excess), 100 °C, 24 h; (d) [Pt(ppy)(μ-Cl)]2, (ppy = 2-phenylpyridinate), NaOAc, NaI, DMSO, 100 °C, overnight; (e) (1) AgBF4, DCM, 1 h, (2) NaCN, rt, overnight.
For 166.2eanti and syn isomers were obtained as a mixture in 1:1 ratio and were not separated. Total yield is given.
Isomers 166.5a and 166.6a were obtained as a mixture in 6:4 ratio and were not separated. Total yield is given.
166.5b and 166.6b were obtained as a mixture in 1:1 ratio and were not separated. Total yield is given.
