Abstract
Background
Clinical management of the muscle spasms and rigidity of tetanus poses a difficult therapeutic problem to physicians everywhere, especially in resource poor countries. There are wide variations in therapeutic regimens commonly used in clinical practice due to uncertainties about effectiveness of conventional drugs. Diazepam compared to other drugs (eg phenobarbitone and chlorpromazine) may have advantages because of combined anticonvulsant, muscle relaxant, sedative and anxiolytic effects.
Objectives
To compare diazepam to other drugs in treating the muscle spasms and rigidity of tetanus in children and adults.
Search methods
We searched the Cochrane Neonatal Group trials register (June 2004), Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, Issue 2, 2004), MEDLINE (1966 to June 2004), EMBASE (1980 to June 2004), LILACS (2004), CINAHL (June 2004), Science Citation Index, African Index Medicus, conference abstracts and reference lists of articles. We contacted researchers, experts and organizations working in the field and used personal communication.
Selection criteria
Randomized and quasi‐randomized controlled trials.
Data collection and analysis
We independently identified eligible trials, assessed trial methodological quality and extracted data.
Main results
Two studies met the inclusion criteria. Method of generation of allocation sequence, concealment of allocation and blinding were unclear in both studies. A total of 134 children were allocated to three treatment groups comprising diazepam alone, phenobarbitone and chlorpromazine, or phenobarbitone and chlorpromazine and diazepam. Meta‐analysis of in‐hospital deaths indicates that children treated with diazepam alone had a better chance of survival than those treated with combination of phenobarbitone and chlorpromazine (Relative Risk for death 0.36; 95% confidence interval 0.15 to 0.86; Risk Difference ‐0.22; 95% CI ‐0.38 to ‐0.06).
Giving diazepam alone, or supplementing conventional anticonvulsants (phenobarbitone and chlorpromazine) with diazepam, was reported in one study to be associated with a statistically significantly milder clinical course and shorter duration of hospitalization.
Authors' conclusions
Although this review suggests that diazepam alone compared with combination of phenobarbitone and chlorpromazine may be more effective in treating tetanus, the small size, methodological limitations and lack of data on drug safety from available trials preclude definite conclusions to support change in current clinical practice. The application of this observation should be moderated by local needs and circumstances, pending the availability of better evidence. We recommend a reinforcement of preventive measures against tetanus infection and it is hoped that in the light of clear evidence about the preventive efficacy of tetanus toxoid immunization, concerted efforts should be made towards preventive interventions and ultimate eradication such that there will not be enough case materials for a trial. In the event of a need for a trial, a large prospective, multicenter, randomized controlled trial, which compares diazepam alone with combinations of other drugs (excluding diazepam) will be ideal.
Plain language summary
Diazepam for treating tetanus
Tetanus is a disease caused by bacteria (Clostridium tetani) found in soil and faeces. It can be immunised against but continues to kill children and adults. Newborn infants are the most vulnerable, particularly in Bangladesh, India, Indonesia, Nigeria and Pakistan, mainly because of unhygienic umbilical cord practices. Puncture wounds, burns, multiple ear piercing, tattooing and circumcision (male and female) can also cause tetanus infection. The symptoms include a sudden onset of muscle stiffness and spasms (involuntary contractions) in the neck, jaw and back, sufficient to cause rigid arching of the back. Glottal and laryngeal spasms may result in fluid being sucked into the breathing passages (aspiration) or inability to breathe (asphyxiation). These spasms progress over two weeks and recovery then takes some four weeks. Complications of the disease or its treatment include depressed breathing, extrapyramidal signs that mimic the tetanus spasms and rigidity, body (autonomic) dysfunction and pneumonia. Supportive nursing, nutritional support and physiotherapy are important. Mechanical ventilation is rarely available in resource poor countries to treat total paralysis. Drugs are needed to reducing the muscle spasms and rigidity, antibiotics to kill the bacteria and tetanus immunoglobulin to remove the toxins in the body. Diazepam has anticonvulsant, muscle relaxant, sedative and anxiety reducing effects. Diazepam treatment was associated with fewer deaths than was treatment with a combination of phenobarbitone and chlorpromazine. Combination treatments with diazepam did not give any further benefit (and may cause harm). The review authors searched the medical literature and identified two randomised controlled trials with a total of 134 hospitalized neonates and older children who had tetanus from Nigeria (19 neonates, seven children aged between one month and 10 years of age) and Indonesia (74 neonates, 34 children aged between three days and 12 years). All drugs were given orally as medications and feeds are usually given via nasogastric tube in the settings where the disease burden is high. Neither study provided information on the safety of the interventions or followed up survivors beyond discharge from hospital.
Background
Tetanus is a potentially fatal disease caused by a potent neurotoxin, tetanospasmin, produced by a bacterium called Clostridium tetani, which is found in soil, human and animal faeces. It is preventable and can be eradicated through immunization and hygienic obstetric practices, but continues to kill children and adults, mostly in low and medium income countries of Asia and sub Saharan Africa. It is estimated that every year 500,000 children and 50,000 adults die from tetanus worldwide (WHO 1996). Neonates are the most vulnerable group, and 80% of cases of neonatal tetanus occur in Bangladesh, India, Indonesia, Nigeria and Pakistan. Mortality and case fatality rates are as high as 28 per 100,000 and 80% respectively in these countries, compared with 0.1% case fatality in developed countries (Stanfield 1984, WHO 1996). Incidence of tetanus in developed countries is as low as 30 to 50 cases per annum, mostly occurring in intravenous drug addicts and elderly adults over 50 years of age, who were either inadequately or never immunized (Levinson 1955, CDC 1999).
Tetanus is transmitted by tetanus spores, which are inoculated into the body following major or minor injuries. In newborn infants, the main route of infection is through the umbilical cord, either because of cutting the cord at birth using contaminated instruments, or through unhygienic cord care practices such as the application of animal dung dressing on the umbilical cord. Acute wounds (including minor splinter punctures), burns, multiple ear piercing, tattooing and circumcision (especially female genital mutilation) may expose non‐immunized individuals to tetanus infection. In 20 to 30% of cases the portal of entry is either obscure or trivial. Following inoculation, the tetanus spores germinate and produce two toxins known as tetanospasmin, which produces the characteristic features and complications of tetanus (i.e. muscle spasms and autonomic dysfunction) and tetanolysin, which causes hemolysis but otherwise plays no major role in the disease (Edsall 1976, Willis 1983, Bleck 1987, Bleck 1991).
The diagnosis of tetanus rests primarily on clinical features. These include sudden onset of muscle stiffness (rigidity) and muscle spasms (involuntary contractions) of the neck and jaw, leading to lockjaw, feeding and speech difficulties and a characteristic facial expression, the sardonic smile (risus sardonicus). Tetanus spasms, also referred to as seizures (fits), may be either localized or generalized. Tonic spasms of muscles of the neck, back, trunk, limbs and abdomen may be associated with rigid arching of the back (opisthotonus). Glottal and laryngeal spasms may develop and these are potentially life‐threatening, as they may lead to aspiration or asphyxiation. Apart from deep sedation that may be associated with use of drugs in tetanus, consciousness level is usually preserved in uncomplicated tetanus. Laboratory tests may be required to exclude other medical conditions that may mimic or complicate tetanus (Bleck 1995, Osinusi 1986, Weinstein 1998). The average duration of the illness is six weeks, comprising a two‐week progression period and another four weeks for recovery, which is usually complete unless complications supervene. Complications of tetanus may be due to the disease or therapeutic interventions, and include respiratory depression, autonomic dysfunction and aspiration pneumonia. Long‐term neurological sequelae have been reported after tetanus disease (Luisto 1989).
Generalized tetanus is the most common and most severe form of the disease. Localized tetanus is usually a mild, self‐limiting illness except when it involves the head and neck (cephalic tetanus). The severity of tetanus is determined by the frequency of spasms, and the presence of opisthotonus and autonomic dysfunction. Various prognostic scoring systems exist (Patel 1959). For example, a scoring index to determine prognosis, at the time of admission and subsequently, was described by Hendrickse (Hendrickse 1981).
Several aspects are involved in the management of tetanus including drug treatment for the muscle spasms and rigidity, the use of antibiotics to kill the bacteria, the administration of tetanus immune globulin to remove free toxins, supportive nursing care, nutritional support, physiotherapy and active immunization with tetanus toxoid. The treatment of tetanus muscle spasms and rigidity is one of the most important aspects as it is crucial to the outcome. This poses a major challenge to physicians in low and middle‐income and even developed countries, as there is no standardized, universally accepted drug regimen for treating tetanus muscle spasms and rigidity. Drugs that have been used for treating tetanus muscle spasms and rigidity include: diazepam, phenobarbitone, chlorpromazine, magnesium sulphate, vecuronium, pancuronium, and less commonly pyridoxine, morphine, baclofen, dantrolene and meprobamate. Wide variations in practice exist. Some tertiary health institutions in countries such as Nigeria use a cocktail of two or more therapeutic agents that include phenobarbitone and chlorpromazine (Kaine 1975, Adedoyin 1982, Oruamabo 1986, Osinusi 1986, Antia‐Obong 1991), while others use diazepam alone (Tompkins 1958, Blankson 1977, Grange 1991, Okuonghae 1992).
DIAZEPAM
Diazepam, a benzodiazepine derivative, has long been reported to be effective in the treatment of tetanus (Weinberg 1964). Subsequently, several other reports suggested that diazepam compared with other therapeutic agents offers significant benefits in the treatment of tetanus (Norredam 1970, Joseph 1978, Alvarado‐Ganoza 1983, Arrate 1980). These benefits are attributed to the combined anticonvulsant and muscle relaxation actions on tetanus muscle spasms and rigidity. Also, it has sedative and anxiolytic effects. Unlike the barbiturates, diazepam, given in high doses, is reported to cause little respiratory depression (Nicol 1967, Fox 1968, Dalen 1969). Diazepam may be given orally via nasogastric tube, per rectum, or by intravenous infusion. Intramuscular diazepam is characterized by erratic absorption. Bioavailability after oral and rectal administration of diazepam is reported to be good, with almost complete absorption and peak plasma levels occurring within 30‐90 minutes (Rey 1981).
OTHER DRUGS
Other drugs that have been used to treat tetanus include phenobarbitone, an anticonvulsant which may have harmful side effects on the respiratory and cardiovascular systems (Norredam 1970, Vassa 1974); chlorpromazine, which in addition to its anticonvulsant effect has muscle relaxant properties but may produce extrapyramidal signs that mimic tetanus spasms and rigidity (Norredam 1970); and magnesium sulphate, found to be most beneficial when used in combination with diazepam, but which may require careful titration and/or measurement of serum magnesium to maintain serum concentrations within the therapeutic range (Cholst 1984, Bleck 1986, Lipman 1987, Attygalle 1997). In addition to the aforementioned, other drugs that have been used in treating tetanus include vecuronium, pancuronium, propofol, baclofen, mephenesin, meprobamate and antitetanus serum. These have various undesirable effects, which include respiratory depression, hepatotoxicity and haemolysis and are mostly not conventional drugs for treating tetanus especially in areas most affected by the disease (Gupta 1979, Bleck 1995, Hajailay 1983, Rocke 1986).
The choice of therapeutic agents used in the treatment of tetanus spasms and rigidity should be based on best evidence of potential benefits and harm, but at best the efficacies of the various agents can be described as controversial. Comparisons of treatment outcomes between published studies have been difficult due to differences in study population and treatment protocols (Patel 1959, Gupta 1979). Many of the reports on beneficial therapeutic effects in tetanus are experiential in nature, based on comparisons between the outcomes of current patients with those of historical controls treated with other drugs. Many of the studies claiming therapeutic benefits are not randomized, controlled trials, which are adjudged the gold standard for best therapeutic evidence. Thus, the question of the benefits and harms of diazepam compared with other pharmacologic treatments such as phenobarbitone and chlorpromazine has remained unresolved.
Although tetanus predominantly affects neonates in the most vulnerable countries, randomized controlled trials based exclusively on neonates may be lacking. Existing studies may include neonates, older children and adults, and this formed the justification for not limiting this review to neonates. Furthermore, studies in which treatments involve total paralysis and mechanical ventilation will be excluded in this review because this mode of treatment is rarely available in resource poor countries most affected by tetanus disease. A separate review on the efficacy of this method of treatment is considered more appropriate.
There is a continuing search for an optimal treatment regime, which is safe, effective, available and affordable, and which controls muscle spasms and rigidity associated with tetanus without requiring any need for artificial ventilation. The aim of this review is to evaluate the existing evidence from controlled clinical trials concerning the benefits and harms of diazepam in the treatment of muscle spasms and rigidity in tetanus.
Objectives
Primary objective
To determine the effects of diazepam compared with other drugs (phenobarbitone, chlorpromazine, magnesium sulphate, pyridoxine, vecuronium, pancuronium) on in‐hospital survival and long‐term sequelae, in patients with tetanus.
Secondary objective
To determine the role of the following on the effects of diazepam: 1. Route of administration ‐ intravenous, rectal or oral 2. Age ‐ neonates (0‐28 days), older children (>1 month ‐ 18 years), adults (>18 years) 3. Local or generalized tetanus at study entry 4. Severity at study entry ‐ mild, moderate, severe, very severe and life threatening, based on the classification by Patel 1959 or as pre‐specified by trialists
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials and quasi‐randomized controlled trials.
Types of participants
Neonates (newborn babies <1 month of age), post‐neonatal children or adults, with clinically confirmed tetanus and admitted into hospital
Definition of tetanus will include localized or generalized types and severity will be categorized into grades I‐V (Patel 1959)
Types of interventions
Intervention: Intravenous, rectal or oral diazepam, in any dose or dose schedule
Control: Phenobarbitone, chlorpromazine, magnesium sulphate, pyridoxine, vecuronium, pancuronium, used either alone or in combination, given parenterally or orally, in any dose or dose schedule
An additional comparison was included post facto, that is diazepam alone versus any other drug plus or minus diazepam
Types of outcome measures
Primary outcomes:
Death
Need for tracheostomy or artificial ventilation
Secondary outcomes:
Time to control muscle spasms (time of commencement of treatment to complete cessation of muscle spasms).
Length of stay in hospital (period from admission to discharge).
Adverse events:
Respiratory depression (age‐related reduction in respiratory rate or cessation of respiration)
Autonomic dysfunction (indicated by instability in systemic blood pressure)
Other adverse events
Search methods for identification of studies
See: Collaborative Review Group Search Strategy We attempted to identify all relevant studies regardless of language or publication status (published, unpublished, in press, and in progress). We searched all trial registers and databases using the search terms: tetanus and diazepam. Search terms included synonyms and trade names of diazepam such as valium, stesolid, seduxen, faustan, diazemuls, and cercin. Chemical names were not be included in the search terms. We searched the Cochrane Neonatal Group, Cochrane Wounds Group and Cochrane Infectious Diseases Group specialized trials registers for relevant trials up to the month of June 2004. We searched The Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, Issue 2, 2004). This contains mainly reference information to randomized controlled trials and controlled clinical trials in health care. We searched the following electronic databases using the topic search terms in combination with search strategy for identifying trials developed by The Cochrane Collaboration and detailed in the Cochrane Reviewers' Handbook (Clarke 2003a): 1) MEDLINE (1966 to June 2004) 2) EMBASE (1980 to June 2004) 3) LILACS (La Literatura Latin americana y del Caribe de information en Ciencias de Salud) 1982 to June 2004. We contacted organizations and individuals who have worked on related research and these included: World Health Organization (Infectious Diseases and Technical Report Groups); University Departments of Paediatrics in areas currently most affected by tetanus (Africa, Asia and India); Medical Research Council, The Gambia; Kenya Medical Research Institute, Clinical Research Centre Kilifi, Kenya; National Institute of Medical Research, Ifakara Centre, Tanzania; Nigeria Institute of Medical Research. We searched conference proceedings and symposia manually for reports of trials and contacted major pharmaceutical companies that formulate diazepam such as Roche. The reviewers consulted existing reviews on the topic, and identified relevant citations. The review protocol panel, internal and external editors, checked the completeness of the search strategy.
Data collection and analysis
Study selection
CO scanned the results of the search strategy, and retrieved full articles for potentially relevant trials. Two reviewers, CO and AL, independently applied the inclusion criteria using the eligibility form based on the contents of the section "criteria for inclusion". We resolved any disagreement through discussion. Studies that did not fulfil the inclusion criteria were excluded and the bases for their exclusion stated in the table "Characteristics of Excluded Studies".
Assessment of methodological quality
We independently assessed studies that met the inclusion criteria for their methodological quality in relation to generation of allocation sequence, allocation concealment, blinding and loss to follow up. For each selected trial, each quality component excluding blinding was categorized as 'adequate', 'inadequate' or 'unclear' according to Juni et al (Juni 2001). For loss to follow up, inclusion of 90% of participants was considered adequate. We assigned each trial a score for concealment of allocation of either A (low risk of bias), B (moderate risk of bias), or C (high risk of bias) as contained in the Cochrane Reviewers' Handbook (Clarke 2003b). Blinding of the intervention to caregivers and blinding of outcome ascertainment were noted. We assessed blinding as open (all parties were aware of treatment), single (the participant or care provider/assessor was aware of the treatment given), or double (trial used a placebo or a double‐dummy technique such that neither the participant or care provider/assessor knew which treatment was given) blind. We were aware that blinding may not have been attempted in trials where different routes of administration were used. We resolved any disagreements through discussion or by consulting a third party. Where information was classed as 'unclear' we attempted to contact the authors. This information was displayed in an additional table and described in the section "Methodological Quality of Included Studies". Following inclusion of all eligible studies, we aimed to conduct sensitivity analysis for each of the quality factors using the above subgroups.
Data extraction
CO and AL independently applied a piloted data extraction form to extract data from selected trials on study characteristics including methods, participants, interventions, and outcomes. Any disagreements between the reviewers was resolved by discussion. Where data from the trial report were either inadequate or missing, we attempted to contact the authors for additional information. Where applicable, we extracted data to allow an intention‐to‐treat analysis. If the numbers randomized and the numbers analyzed were inconsistent, the percentage loss‐to‐follow‐up was to be calculated and this information reported in an additional table. For binary outcomes we recorded the number of participants experiencing the event in each group of the trial. For continuous outcomes for each group, we extracted the arithmetic means and standard deviations. For data reported using geometric means, standard deviations were to be extracted on the log scale. If provided in the trial, medians and ranges were extracted and reported in tables.
Data analysis
CO and AL analyzed the data using Review Manager (Version 4.2). Outcome measures for binary data were compared using the relative risk (RR) and its 95% confidence interval; for continuous data, the weighted mean difference (WMD) and its 95% confidence interval. Where continuous data had been reported using geometric means, the findings were to be combined on a log scale and reported on the original scale. Medians and ranges were to be reported in tables only. Subgroup analyses were to be carried out to determine the role of age, pattern of tetanus (localized or generalized), severity at study entry, and route of administration on the effects of diazepam. For meta‐analyses, we used a fixed effect model.
Additional comparisons and analyses were added post facto because the two eligible studies included these groups. These were: 1) Diazepam alone versus phenobarbitone and chlorpromazine and diazepam 2) Diazepam alone versus phenobarbitone and chlorpromazine with or without diazepam
Results
Description of studies
Eligibility
We identified 12 potentially relevant publications, of which two met the inclusion criteria (Hendrickse 1965, Tjoen 1970). We have provided reasons for excluding 10 other studies in the characteristics of excluded studies. The main reasons for exclusion were non‐randomization of comparison groups, non‐inclusion of a diazepam alone group and non‐inclusion of a control group.
Location
The two included studies were conducted in Nigeria (Hendrickse 1965) and Indonesia (Tjoen 1970) located in sub‐Saharan Africa and South East Asia regions, where the tetanus burden remains notably high.
Participants
The trials studied a total of 134 participants comprising hospitalized neonates and older children with tetanus. Hendrickse 1965 studied 26 (19 neonates, seven post‐neonatal) Nigerian children aged between one month and 10 years of age, while Tjoen 1970 studied 108 (74 neonates, 34 post‐neonatal) Indonesian children aged between three days and 12 years. Adults were excluded in both studies. Hendrickse 1965 did not pre‐specify the severity of tetanus at study entry, while Tjoen 1970 categorized severity of tetanus at recruitment as severe, moderate and mild.
Interventions
The two eligible studies allocated the participants to three similar groups 1) Diazepam alone (experimental intervention ) 2) Phenobarbitone and chlorpromazine (control intervention A) 3) Phenobarbitone, chlorpromazine and diazepam (control intervention B)
In Hendrickse 1965, a total of eight children (five neonates, three older children) were randomly allocated to diazepam alone group, while nine children each (seven neonates, two older children) were allocated the control groups A and B respectively. In Tjoen 1970, 33 children excluding neonates were allocated to the diazepam alone group, while 38 and 37 children were allocated to control groups A and B respectively. The reason for excluding all neonates from the experimental intervention was described as due to 'bad experience' with diazepam in the older children. The numbers of neonates allocated to control groups A and B were not stated. The experimental and control drugs were administered by the oral route in both trials. Overall, the doses of both the experimental and control interventions were higher in Tjoen 1970, compared to Hendrickse 1965. As much as 4‐9 mg/kg diazepam was used in Tjoen 1970 compared with 0.44 to 1.1mg/kg in Hendrickse 1965.
Participants in both studies received other standard treatments for tetanus, which included intramuscular phenobarbitone and chlorpromazine given on admission to all the participants in Tjoen 1970, to control spasms and convulsions. Intramuscular paraldehyde was used for the same purpose in Hendrickse 1965. Neither of the studies reported on the role of nursing care in the standard management of tetanus.
Outcome measures
Both trials reported in‐hospital, all‐cause death as primary outcome. Tjoen 1970 reported mortalities according to author pre‐specified categories of severity of tetanus (severe and mild to moderate). Other outcome measures in Tjoen 1970 included duration of abdominal wall spasm, trismus and tonic convulsions, length of hospitalization, and time to regain motor activities (sitting up, standing up and walking).
Adverse events
The two included studies did not pre‐specify adverse events or complications to be measured in survivors or prior to death; however in Tjoen 1970, death‐related complications (hyperpyrexia, aspiration pneumonia and bronchopneumonia) were reported. Hendrickse 1965 reported on one case of fatal bronchopneumonia.
Risk of bias in included studies
The two included trials were randomised (Hendrickse 1965) and quasi‐randomised (Tjoen 1970) controlled trials. Tjoen 1970 used alternation, but the method of generation of allocation sequence and the adequacy of concealment of allocation were unclear in both studies. Blinding of the interventions to caregivers and blinding of outcome measurements were not stated in the trials and these could not be clarified from the authors. Both trials performed an intention to treat analysis. Three children were excluded in Tjoen 1970, of which two were due to incompleteness of records and one left on admission for no stated reason. These exclusions appeared to be prior to randomization into the intervention groups. Data on complications and other adverse events were not adequately provided. See Table 1 for details. We performed meta‐analysis based on reported evaluable participants.
1. Mean clinical course in days (Tjoen 1970).
| Clinical course | Control Group A | Control Group B | Diazepam |
| Spasm of abdominal wall | 6.3 | 2.8 | 2.6 |
| Trismus | 9.9 | 6.0 | 5.3 |
| Tonic convulsion | 3.5 | 1.5 | 2.0 |
| Time to regain motor function | |||
| Sitting up | 3.2 | 0.5 | 1.0 |
| Standing up | 4.9 | 1.2 | 1.5 |
| Walking | 6.0 | 1.5 | 2.6 |
| Hospital stay | 13.0 | 8.1 | 7.0 |
Effects of interventions
Three comparison groups were analysed, including: 1) Diazepam alone versus phenobarbitone and chlorpromazine 2) Diazepam alone versus phenobarbitone and chlorpromazine and diazepam 3) Diazepam alone versus phenobarbitone and chlorpromazine with or without diazepam
Comparisons 2 and 3 were added post facto, because the two eligible studies, Hendrickse 1965 and Tjoen 1970, included diazepam in one of their control groups.
Death (in‐hospital, all cause)
Both trials, Hendrickse 1965 and Tjoen 1970, assessed effect on death.
Diazepam alone versus phenobarbitone and chlorpromazine There was a total of 5/41 deaths in the experimental groups and 16/47 deaths in the control groups. This effect on mortality was consistent in both studies and was statistically significant in the meta‐analysis (Relative Risk 0.36, 95% confidence interval 0.15 to 0.86; Risk Difference ‐0.22, 95% CI ‐0.38 to ‐0.06).
Diazepam alone versus phenobarbitone and chlorpromazine and diazepam In the two studies, addition of diazepam to the control interventions was associated with a total of 5/41 deaths in the diazepam alone groups compared with 10/46 mortalities in the control groups, but this effect was not statistically significant (Relative Risk 0.56; 95% confidence interval 0.22 to 1.45; Risk Difference ‐0.10; 95% confidence interval ‐0.24 to 0.05). This effect was similar across the two included trials.
Diazepam alone versus phenobarbitone and chlorpromazine with or without diazepam In the two studies, the total for deaths in the experimental groups were 5/41 compared with 26/93 in the control groups, and this effect was of borderline statistical significance (Relative Risk 0.44, 95% confidence interval 0.18 to 1.02; Risk Difference ‐0.16, 95% CI ‐0.29, ‐0.03). This effect was similar across the two studies. Hendrickse 1965 noted that the two deaths in the diazepam alone group were following the addition of phenobarbitone and chlorpromazine to one patient, and measles infection in the other patient).
We did not detect any statistically significant heterogeneity of treatment effect in any of the comparisons, suggesting that overall, the two included trials were similar in terms of populations, interventions, outcomes, quality and the direction and magnitude of treatment effects.
There were insufficient data in both trials to do sub‐group analyses to estimate the role of age of participants, pattern of tetanus (localized or generalized), severity of tetanus at study entry, dose or route of administration of interventions.
Other outcomes
Mean clinical course in days (Tjoen 1970) The following other outcomes were reported by Tjoen 1970 (see additional table): 1) Duration of abdominal wall spasm The authors reported a mean duration of 2.6 days, 2.8 days and 6.3 days in the diazepam alone group, and in the control groups B and A respectively.
2) Duration of trismus Mean duration for this outcome was 5.3 days, 6.0 days and 9.9 days in diazepam alone group and control groups B and A respectively.
3) Duration of tonic convulsion Average duration for this outcome was 2.0 days, 1.5 days and 3.5 days in the diazepam alone group and control groups B and A respectively.
4) Time to regain motor function Mean time for sitting up was 1.0, 0.5 and 3.2 days; for standing up 1.5, 1.2, and 4.9 days; and for walking 2.6, 1.5, and 6.0 days in the diazepam alone group and control groups B and A respectively.
5) Length of hospital stay Mean duration of hospitalization was 7.0, 8.1, and 13.0 days in the diazepam group and control groups and control groups B and A respectively.
The authors did not provide other data (standard deviation of means) to permit statistical analysis in this review. The authors noted that supplementing conventional anticonvulsants (phenobarbitone and chlorpromazine) with diazepam, or giving diazepam alone, was associated with statistically significant milder clinical course and shorter duration of hospitalization. However, since they did not report standard deviations, we could not perform statistical analyses in this review.
Data provided on time to death in the two trials were insufficient for any statistical analysis.
Adverse events
Tjoen 1970 reported fatal complications consisting of hyperpyrexia, aspiration pneumonia and bronchopneumonia in seven cases of severe tetanus (three in group A and four in group B). Hendrickse 1965 reported one case of severe fatal bronchopneumonia in control intervention group B. No adverse event was noted in the experimental (diazepam alone) group. The one case of fatal measles reported in this group in Hendrickse 1965 was not related to tetanus and was noted to have occurred after recovery from tetanus. Neither study followed up survivors beyond discharge from hospital to determine disabilities associated with tetanus and the type of interventions used.
We did not find data on other adverse events (eg need for tracheostomy or ventilation support) pre‐specified in the protocol.
Discussion
This review aimed to determine from reliable research the efficacy and safety of diazepam compared to other treatment options used in tetanus. The two included studies were small and the logistics of randomization, in particular generation of allocation sequence and concealment of allocation, were not clear. Blinding of intervention to the caregivers or to the investigators and outcome assessors was not stated. These methodological issues potentially increased the risk of bias, and cast doubt on the internal validity of the trial results. However, while we recognize the difficulties earlier trialists in medical research may have faced in general, it is noteworthy that there are no recent trials of adequate methodological quality based on the eligibility criteria for inclusion, particularly from regions that bear the burden of tetanus. Furthermore, the inclusion of diazepam in one of the two control groups in each of the two included trials "contaminated" those control groups, so that any true effect of diazepam on death and other outcomes may have been diluted by this experimental design.
Noteworthy concerns in this review include the comparability of the study groups that included largely neonates and fewer older children and adults, the bioavailability of oral medications in sick neonates, and the huge difference in the dose of diazepam used in the two studies, which varied by as much as nine fold. Rather than any differences in biological constitution, pathogenesis and pathophysiology of tetanus between neonates, older children and adults, it is the low passive immunity due to inadequate maternal immunization, unhygienic cord care practices, and principal portal of entry by the umbilicus that are the major risk factors unique to neonates. These factors account for their greater predisposition to tetanus compared with other groups. Nevertheless, the effect of a predominantly neonatal age group in the trials may be more evident and possibly change the findings once we diaggregate the two groups, but doing this may further dilute and weaken the power of the studies and findings. Doubts about the bioavailability of medications administered orally in sick neonates will be justified where the sickness involves and/or affects the gastrointestinal tract, in which case there will be clinical pointers supporting same such as abdominal distension, vomiting and/or diarrhoea. In such situations, absorption of gastrointestinal contents including medications may be impaired. Usually, unless tetanus is complicated by septicaemia with gastrointestinal involvement, medications and feeds are usually given via nasogastric tube in the settings where the disease burden is high.
Although there is a consistent beneficial effect in both Hendrickse 1965 and Tjoen 1970 suggesting that diazepam given alone that diazepam alone is more efficacious in treating tetanus compared with other conventional treatment, and that combination therapy may be harmful, this observation is methodologically and statistically limited, and insufficient to support or justify a change in current practice. Also, there were no data on safety of the interventions used in both trials.
Giving diazepam alone or supplementing conventional anticonvulsants (phenobarbitone and chlorpromazine) with diazepam, was reported to be associated with statistically significant milder clinical course and shorter duration of hospitalization in one study.
Applicability:
Location Data included in this review are from studies conducted in areas that still bear the burden of tetanus and these include Nigeria in sub‐Saharan Africa and Indonesia in South East Asia. These resource poor countries still grapple with the burden of tetanus, cannot afford treatments requiring total paralysis and ventilation support as is the case in developed countries, and therefore still depend on the interventions under review. They have the greatest need for evidence of effects of drugs used in treating tetanus.
Populations The two trials enrolled neonates (who are the most affected by tetanus) as well as post‐neonatal children, but not adults. However, the studies failed to stratify randomisation or report outcome data according to age; but we recognize that the smallness of sample size in the trials, particularly in Hendrickse 1965, could have precluded stratification into subgroups.
Benefits and risks Both trials show that diazepam alone is associated with fewer deaths and that additional drugs or combination treatment are not beneficial and may cause harm in tetanus. However, these studies are dated and are insufficient methodologically and statistically for any definite conclusion on benefits. Also, both trials did not provide data of safety of the interventions.
Authors' conclusions
Implications for practice.
Diazepam alone compared to other currently used drugs may be more effective in treating tetanus, and adding other drugs to diazepam has no benefit or may be harmful. However, available data are insufficient and too dated to permit any definite or firm deductions to support change in current practice. Furthermore, these trials were not designed with adverse events as primary outcomes and this further limits any conclusion that may influence current practice. Therefore, the application of the present beneficial effect associated with diazepam should be with caution and be moderated by local needs and circumstances pending the availability of best evidence from large randomized controlled trials.
Implications for research.
This review clearly demonstrates the paucity of recent randomized trials of adequate methodological quality on the benefits and harms of major therapeutic interventions used for treating tetanus, and reinforce the need for further investigations in this area. While a large prospective multi‐center trial may be warranted, it is hoped that in the light of clear evidence about the preventive efficacy of tetanus toxoid immunization, concerted efforts should be reinforced towards preventive interventions and ultimate eradication of tetanus such that there will not be enough case materials for a trial of this nature. However, in the event that a trial becomes absolutely necessary, the flaws of existing trials should be prevented in any future trial and adequate attention should be paid to randomization, blinding and the types of experimental and control interventions given. Also, future trialists should endeavour to state the study objectives and inclusion criteria explicitly; categorize participants by severity of disease; use other clinically pragmatic outcomes; record adverse events. Trial reports should conform to the CONSORT (Consolidated Standards of Reporting Trials) statement to permit the reader to clearly understand the trial design, conduct, analysis, interpretation and report (Moher 2003).
What's new
| Date | Event | Description |
|---|---|---|
| 15 September 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 1, 2003 Review first published: Issue 1, 2004
| Date | Event | Description |
|---|---|---|
| 30 June 2004 | New search has been performed | This updates the existing review "Diazepam for treating tetanus" published in The Cochrane Library, Disk Issue 1, 2004 (Okoromah 2004). No new studies were identified in searches to June 2004. |
Acknowledgements
The protocol for this review was developed during the Mentorship Programme organized by the Cochrane Infectious Diseases Group, July 2002. The Department of International Development (UK) supports this programme through the Effective Health Care Alliance Programme at the Liverpool School of Tropical Medicine.
Data and analyses
Comparison 1. Diazepam alone versus phenobarbitone and chlorpromazine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Deaths (in‐hospital, all‐cause) | 2 | 88 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.15, 0.86] |
1.1. Analysis.
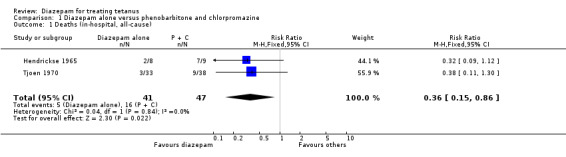
Comparison 1 Diazepam alone versus phenobarbitone and chlorpromazine, Outcome 1 Deaths (in‐hospital, all‐cause).
Comparison 2. Diazepam alone versus phenobarbitone and chlorpromazine and diazepam.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Deaths (in‐hospital, all‐cause) | 2 | 87 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.22, 1.45] |
2.1. Analysis.
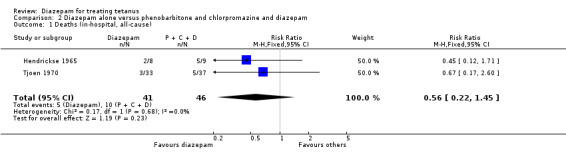
Comparison 2 Diazepam alone versus phenobarbitone and chlorpromazine and diazepam, Outcome 1 Deaths (in‐hospital, all‐cause).
Comparison 3. Diazepam alone versus phenobarbitone and chlorpromazine with or without diazepam.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Deaths (in‐hospital, all‐cause) | 2 | 134 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.18, 1.02] |
3.1. Analysis.
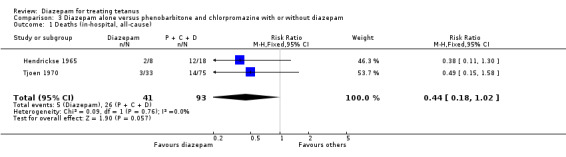
Comparison 3 Diazepam alone versus phenobarbitone and chlorpromazine with or without diazepam, Outcome 1 Deaths (in‐hospital, all‐cause).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Hendrickse 1965.
| Methods | Randomized controlled trial Blinding of allocation (No) Blinding of intervention (No) Blinding of outcome (No) Reported all‐cause mortality Complete follow up (Unclear) |
|
| Participants | Sample size: 26 Neonates: 19 Older children: 7 Age range: 1 month to 10 years | |
| Interventions | Experimental:
Diazepam only
Dose: (0.44 to 1.1mg/kg)
6 hourly
Route: Oral
No. allocated: 8 Controls: (1) Group A Phenobarbitone and Chlorpromazine Dose: (4.4 to 6.6 mg /kg) and (1.1 to 2.2 mg/kg) 6 hourly Route: Oral No. allocated: 9 (2) Group B Phenobarbitone and Chlorpromazine and Diazepam Dose: (4.4 to 6.6 mg /kg) and (1.1 to 2.2 mg/kg) and (0.44‐1.1 mg/kg/) 6 hourly Route: Oral No. allocated: 9 |
|
| Outcomes | Deaths
(1) In‐hospital, directly related to tetanus:
Diazepam: 1
Control group A: 7
Control group B: 5 (2) In‐hospital, related to other causes Diazepam: 1 Control group A: 0 Control group B: 0 Total deaths: 14/26 |
|
| Notes | Study location: Ibadan, Nigeria. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Tjoen 1970.
| Methods | Quasi‐ randomized controlled trial. Blinding of allocation (No) Blinding of intervention (No) Blinding of outcome (No) Complete follow up (Unclear) | |
| Participants | Sample size: 108 Neonates: 33 Older children: 75 Age range: 3 days to 12 years | |
| Interventions | Experimental:
Diazepam only
Dose: (1.5 to 9.0 mg /kg) per day
No. allocated: 33 (excluded neonates) Controls (1) Group A Phenobarbitone and Chlorpromazine Dose: (100‐200 mg/day) and (2 to 4 mg/day) Route: Oral No. allocated: 39 (2) Group B Phenobarbitone and Chlorpromazine and Diazepam Dose: (100 to 200 mg/day) and (2 mg to 4 mg/ kg/day) and (1.5 mg to 9.0 mg/kg/day) Route: Oral No. allocated: 37 |
|
| Outcomes | Deaths
(1) In‐hospital,
directly related to tetanus:
Diazepam: 3
Control group A: 8
Control group B: 2 (2) In‐hospital, related to other causes Diazepam: 3 Control group A: 1 Control group B: 3 Total deaths: 17/108 |
|
| Notes | Study location: Jakarta, Indonesia. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bhandari 1980 | Not randomized controlled trial and no diazepam alone treatment group. |
| Daud 1981 | Randomized controlled trial but no diazepam alone treatment group. |
| Femi‐Pearse 1966 | Not randomized controlled trial. |
| Hendrickse 1966 | Part of continuation of a preliminary randomized study conducted in 1965, but diazepam only group was excluded. |
| Husada 1976 | Not randomized and no diazepam alone group. Diazepam was given to all treatment groups. |
| Joseph 1978 | Not randomized controlled trial. |
| Keswan 1983 | Not ramdomized controlled trial. |
| Norredam 1970 | Not randomized controlled trial. |
| Sugitha 1983 | Not randomized controlled trial. |
| Vassa 1974 | Not randomized and no diazepam alone treatment group. |
Contributions of authors
Christy Okoromah (CO) and Afolabi Lesi (AL) initiated the topic. CO developed the protocol, designed the eligibility and validity criteria, and the data extraction forms. CO and AL modified the data extraction form, extracted the data and analysed the data. CO wrote the results and discussion and AL revised them.
Sources of support
Internal sources
Liverpool School of Tropical Medicine, UK.
College of Medicine of the University of Lagos, Nigeria.
External sources
Department for International Development, UK.
Declarations of interest
We certify that we have no affiliations with or involvement in any organization or entity with a direct financial interest in the subject matter of this review (eg employment, consultancy, stock ownership, honoraria, and expert testimony).
Edited (no change to conclusions)
References
References to studies included in this review
Hendrickse 1965 {published data only}
- Hendrickse RG, Sherman PM. Tetanus in childhood: report of a therapeutic trial of diazepam. British Medical Journal 1966;2:860‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tjoen 1970 {published data only}
- Tjoen LW, Darmawan S, Ismael S, Sudigbia I, Suradi R, Munthe BG. The effect of diazepam on tetanus. Paediatrica Indonesiana 1970;10:248‐58. [PubMed] [Google Scholar]
References to studies excluded from this review
Bhandari 1980 {published data only}
- Bhandari NR, Shrivastava V. A study of tetanus neonatorum: different regimens of treatment. Indian Paediatrics 1980;17:803‐8. [PubMed] [Google Scholar]
Daud 1981 {published data only}
- Daud S, Mohammad T, Ahmad A. Tetanus neonatorum. A preliminary report on the assessment of different therapeutic regimens. Journal of Pakistan Medical Association 1981;31:105‐8. [PubMed] [Google Scholar]
Femi‐Pearse 1966 {published data only}
- Femi‐Pearse D. Experience with diazepam in tetanus. British Medical Journal 1966;2:862‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hendrickse 1966 {published data only}
- Hendrickse RG, Sherman PM. Tetanus in childhood: report of a therapeutic trial of diazepam. British Medical Journal 1966;2:860‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Husada 1976 {published data only}
- Husada T, Rampengan TH, Harjanto IG, Arif IG, Munir M. Neonatal tetanus. Evaluation of treatment and a proposal for classification of severity. Paediatrica Indonesia 1976;16:345‐54. [PubMed] [Google Scholar]
Joseph 1978 {published data only}
- Joseph A, Pulimood BM. Use diazepam in tetanus: a comparative study. Indian Journal of Medical Research 1978;68:489‐91. [PubMed] [Google Scholar]
Keswan 1983 {published data only}
- Keswan NK, Singh AK, Singh DR. Continuous intravenous therapy in severe tetanus. Journal of Indian Medical Association 1983;81:64‐5. [Google Scholar]
Norredam 1970 {published data only}
- Norredam K, Hainau B. Treatment of tetanus in tropical Africa: a comparison between barbiturate and diazepam in the treatment of non‐neonatal tetanus. Annals of Society of Belgium Tropical Medicine 1970;50:239‐46. [PubMed] [Google Scholar]
Sugitha 1983 {published data only}
- Sugitha N, Suwendra P, Suraatmaja SA. High dosage diazepam as single antispasmodic agent in the treatment of neonatal tetanus. Paediatrica Indonesia 1983;23:163‐72. [PubMed] [Google Scholar]
Vassa 1974 {published data only}
- Vassa NT, Doshi HV, Yajnik VH, Shah SS, Joshi KR, Patel SH. Comparative clinical trial of diazepam with other conventional drugs in tetanus. Postgraduate Medical Journal 1974;50:755‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Abrutyn 1991
- Abrutyn E, Berlin JA. Intrathecal therapy in tetanus: A meta‐analysis. Journal of the American Medical Association 1991;266:2262‐7. [PubMed] [Google Scholar]
Adedoyin 1982
- Adedoyin HA, Kadri DO. Neonatal tetanus in IIorin. Nigerian Medical Journal 1982;12:348‐50. [Google Scholar]
Alvarado‐Ganoza 1983
- Alvarado‐Ganoza G, Bermejo‐Sanchez F, Morales‐Moreno R, Kawano‐Nakamura J. Tetanus neonatorum: evaluation of 4 therapeutic regimens. Boletin medico del Hospital Infantil de Mexico 1983;40:251‐5. [PubMed] [Google Scholar]
Antia‐Obong 1991
- Antia‐Obong OE, Ikpatt HW. Neonatal tetanus in Calabar: A 4‐year retrospective study. Nigerian Journal of Paediatrics 1991;18:44‐8. [Google Scholar]
Arrate 1980
- Arrate JK, Ugidos M, Garcia Rodrigo. Neonatal tetanum treatment with high doses of diazepam. Espanoles de Pediatria 1980;13:243‐6. [Google Scholar]
Attygalle 1997
- Attygalle D, Rodrigo N. Magnesium sulphate for the control of spasms in severe tetanus. Can we avoid sedation and artificial ventilation?. Anaesthesia 1997;52:956‐62. [DOI] [PubMed] [Google Scholar]
Billimoria 1981
- Billimoria RB, Chhabra RB, Satoskar RB. Evaluation of diazepam alone and in combination with chlorpromazine or propranolol in the therapy of tetanus. Journal of Postgraduate Medicine 1981;27:80‐5. [PubMed] [Google Scholar]
Blankson 1977
- Blankson JM. Problems of neonatal tetanus as seen in Ghana. African Journal of Medical Science. 1977;6:7‐13. [PubMed] [Google Scholar]
Bleck 1986
- Bleck TP. Pharmacology of tetanus. Clinical Neuropharmacology 1986;9:103‐20. [DOI] [PubMed] [Google Scholar]
Bleck 1987
- Bleck TP. Tetanus: Dealing with the continuing clinical challenge. The Journal of Critical IIlness 1987;2:41‐52. [Google Scholar]
Bleck 1991
- Bleck TP. Tetanus: pathophysiology, management, and prophylaxis. Disease‐a‐month 1991;37:545‐603. [DOI] [PubMed] [Google Scholar]
Bleck 1995
- Bleck TP. Clostridium Tetani. Mandell Douglas and Bernett's Principles and Practice of Infectious Diseases. 4. Vol. 2, Melbourne: Churchill Livingstone, 1995:2173‐8. [Google Scholar]
CDC 1999
- Centers for Disease Control. Tetanus: United States, 1987 and 1988. Morbidity and Mortality Weekly Report (MMWR) 1999;39:37‐41. [PubMed] [Google Scholar]
Cholst 1984
- Cholst IN, Steinberg SF, Tropper PJ, Fox HE, Segre GV, Bilezkian JP. The influence of hypermagnesemia on serum calcium and parathyroid hormone levels in human subjects. New England Journal of Medicine 1984;310:1221‐5. [DOI] [PubMed] [Google Scholar]
Clarke 2003a
- Clarke M, Oxman A, editors. Optimal search strategy. Cochrane Reviewers Handbook 4.2 (updated March 2003); Appendix 5c. The Cochrane Library 2003, Issue 3. [Google Scholar]
Clarke 2003b
- Clarke M, Oxman A, editors. Approaches to summarising the validity of studies. Simple approaches. Cochrane Reviewers Handbook 4.2 (updated March 2003); Section 6.7.1. The Cochrane Library 2003, Issue 3. [Google Scholar]
Dalen 1969
- Dalen JE, Evans GL, Banas JS, Brooks HL, Paraskos JA, Dexter L. The haemodynamic and respiratory effects of diazepam (Valium). Anesthesiology 1969;30:259‐63. [DOI] [PubMed] [Google Scholar]
Edsall 1976
- Edsall G. Problems in the immunology and control of tetanus. Medical Journal of Australia 1976;2:216‐20. [DOI] [PubMed] [Google Scholar]
Fox 1968
- Fox GS, Wynands JE, Bhambhami M. A clinical comparison of diazepam and thiopentone as induction agents to general anaesthesia. Canadian Anaesthetists' Society Journal 1968;15:281‐90. [DOI] [PubMed] [Google Scholar]
Gowers 1888
- Gowers WR. A manual of diseases of the nervous system. Philadelphia: Blackiston, 1888. [Google Scholar]
Grange 1991
- Grange AO. Neonatal tetanus in Lagos Metropolis. Nigerian Journal of Paediatrics 1991;18:12‐22. [Google Scholar]
Grewal 1969
- Grewal RS, Sharma BK. Valium in the treatment of tetanus. Indian Practitioner 1969;22:643. [Google Scholar]
Gupta 1979
- Gupta SM, Takkar VP, Verma AK. A retrospective study of tetanus neonatorum and comparative assessment of diazepam in its treatment. Indian Pediatrics 1979;16:343‐7. [PubMed] [Google Scholar]
Hajailay 1983
- Hajailay R, Sharan R, Agarwal VK, Srivastava AK. Pyridoxine therapy in tetanus neonatorum. Indian Pediatrics 1983;20:935‐9. [PubMed] [Google Scholar]
Hendrickse 1981
- Hendrickse RG. Paediatrics in the tropics: current review. Oxford: Oxford University Press, 1981. [Google Scholar]
Juni 2001
- Juni P, Altman DG, Egger M. Systematic reviews in health care: Assessing the quality of controlled clinical trials. British Medical Journal 2001;323:42‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaine 1975
- Kaine W. A review of neonatal tetanus in Enugu. Nigerian Journal of Medicine 1975;5:108‐10. [Google Scholar]
Levinson 1955
- Levinson A, Marska RL, Shein MK. Tetanus in heroin addicts. Journal of the American Medical Association 1955;157:658‐60. [DOI] [PubMed] [Google Scholar]
Lipman 1987
- Lipman J, James MFM, Erskine J, Plit ML, Eidelman J, Esser JD. Autonomic dysfunction in severe tetanus: Magnesium sulfate as an adjunct to deep sedation. Critical Care Medicine 1987;15:987‐8. [PubMed] [Google Scholar]
Luisto 1989
- Luisto M. Outcome and neurological sequelae of patients after tetanus. Acta Neurologica Scandinavica 1989;80:504‐511. [DOI] [PubMed] [Google Scholar]
Moher 2003
- Moher D, Schultz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel‐group randomised trials. Clinical Oral Investigations 2003;7:2‐7. [DOI] [PubMed] [Google Scholar]
Nicol 1967
- Nicol CF, Tutton JC. The role of diazepam in the treatment of status epilepticus. Neurology 1967;17:281‐3. [Google Scholar]
Okuonghae 1992
- Okuonghae HO, Airede AI. Neonatal tetanus: incidence and improved outcome with diazepam. Developmental Medicine and Child Neurology 1992;34:448‐53. [DOI] [PubMed] [Google Scholar]
Oruamabo 1986
- Oruamabo RS, Mbagbaw IT. Neonatal tetanus in Port Harcourt. Nigerian Journal of Paediatrics 1986;13:115‐20. [Google Scholar]
Osinusi 1986
- Osinusi K, Dawodu AH, Sodeinde O, Adeyokunnu AA. Neonatal tetanus in Ibadan. Nigerian Journal of Paediatrics 1986;13:212‐15. [Google Scholar]
Osinusi 1997
- Osinusi K, Nyinyam MN. A new prognostic scoring system in neonatal tetanus. African Journal of Medical Science 1997;26:123‐5. [PubMed] [Google Scholar]
Patel 1959
- Patel JC, Joag GG. Grading of tetanus to evaluate prognosis. Indian Journal of Medical Science 1959;13:834‐6. [PubMed] [Google Scholar]
Paul 1984
- Paul SS, Utal DS, Jana AK, Mathew J. Therapy in neonatal tetanus. Indian Pediatrics 1984;21:689‐94. [PubMed] [Google Scholar]
Rey 1981
- Rey M, Diop‐Mar I, Robert D. Tetanus: Important new concepts. In: Veronesi R editor(s). Excerpta Medica. Amsterdam, 1981:207‐37. [Google Scholar]
Rocke 1986
- Rocke DA, Wesley AG, Pather M, Calver AD, Hariparsad D. Morphine in tetanus ‐ the management of sympathetic nervous system overactivity. South African Medical Journal 1986;70:666‐8. [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. Journal of American Medical Association 1995;273:408‐12. [DOI] [PubMed] [Google Scholar]
Stanfield 1984
- Stanfield JP, Galazka A. Neonatal tetanus in the world today. Bulletin of the World Health Organization 1984;62:647‐69. [PMC free article] [PubMed] [Google Scholar]
Tompkins 1958
- Tompkins AB. Neonatal tetanus in Nigeria. British Medical Journal 1958;11:382‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weinberg 1964
- Weinberg WA. Control of the neuromuscular and convulsive manifestations of severe systemic tetanus: case report with a new drug Valium (Diazepam). Clinical Pediatrics 1964;71:226‐8. [DOI] [PubMed] [Google Scholar]
Weinstein 1998
- Weinstein LL, Harrison RE, Cherry JD. Tetanus. In: Feigin, Cherry editor(s). Textbook of paediatric infectious diseases. 4. Vol. 4, Philadelphia: W.B. Saunders Company, 1998:1577‐87. [Google Scholar]
WHO 1996
- World Health Organization. Total tetanus incidence data. Bulletin of the World Health Organization 1996;70:11‐17. [Google Scholar]
Willis 1983
- Willis AT. Topley and Wilsons principles of bacteriology, virology and immunity. Vol. 2, Baltimore: Williams and Wilkins, 1983. [Google Scholar]
References to other published versions of this review
Okoroma 2004
- Okoromah CAN, Lesi FEA. Diazepam for treating tetanus. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD003954.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


