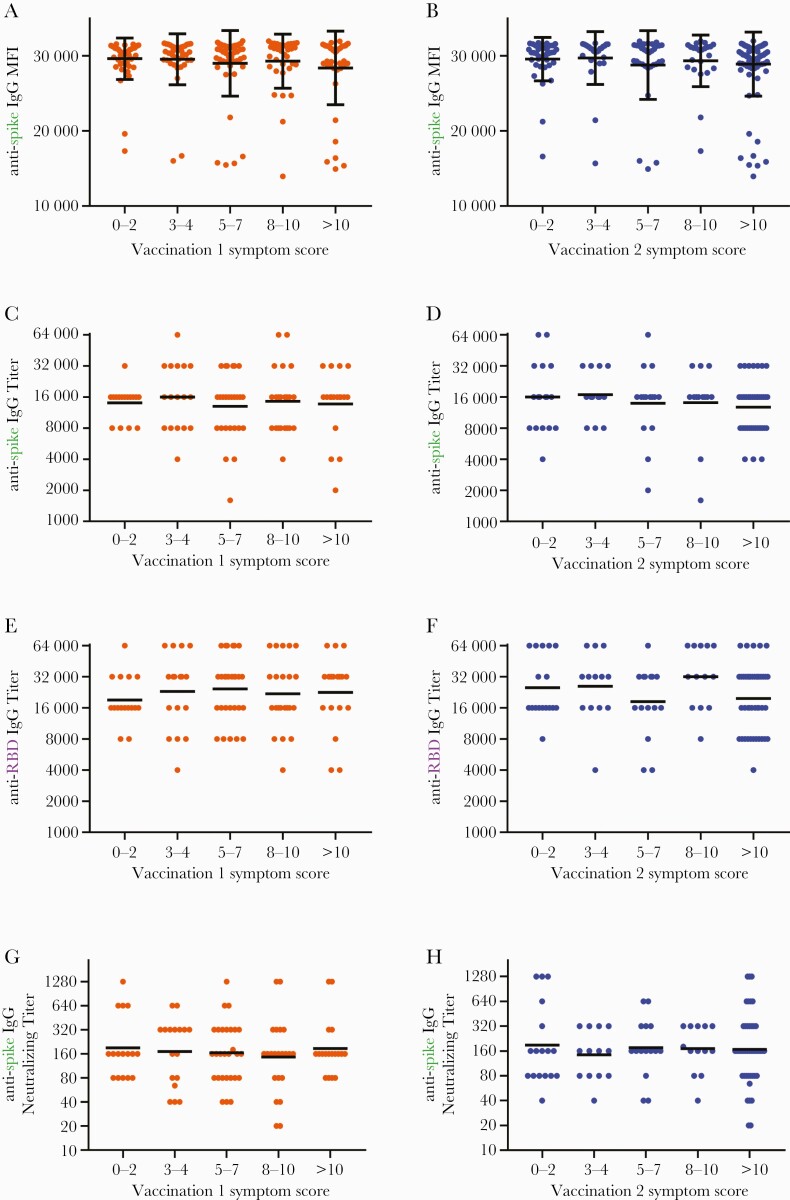
Figure 4.
Severity of symptoms after vaccination correlates with neither vaccine-induced antispike immunoglobulin (Ig)G reactivity nor with titers of antispike and anti-receptor-binding domain (RBD) IgG antibodies. Samples collected ~1 month (mean 36.8 days for A-B and 33.9 days for C-H) after participants received the second vaccine dose of BNT162b2 were tested for antibodies against SARS-CoV-2 spike and RBD proteins using the Luminex microsphere-based multiplex immunoassay. (A and B) Levels of antispike IgG antibodies, as measured by mean fluorescence intensity (MFI), were plotted against symptom scores reported after first (orange) and second (blue) vaccination (N = 206). (C and D) Titers of antispike IgG antibodies, (E and F) anti-RBD IgG antibodies, and (G and H) neutralizing antibodies were plotted against symptom scores reported after first and second vaccination (n = 101). Bars indicate mean and standard deviation (A and B) or geometric mean (C–F). Titers recorded as >32000 are plotted as 64000. Assessments for correlations were conducted by both Kruskal-Wallis analysis with subjects binned into categories of symptom score ranges and by Spearman rank analysis evaluating antibody levels against symptom scores as a continuous variable. All showed no significant correlations.