Abstract
Introduction:
Microglia are the resident tissue macrophages of the central nervous system. Prolonged microglial activation often occurs after traumatic brain injury, and is associated with worsening neurocognitive outcomes. Resolution of microglial activation is associated with limited tissue loss and improved neurocognitive outcomes. Limiting the prolonged pro-inflammatory response and the associated secondary tissue injury provides the rationale and scientific premise for considering microglia as a therapeutic target.
Areas Covered:
In this review, we discuss markers of microglial activation, like immunophenotype, and microglial response to injury, including cytokine/chemokine release, free radical formation, morphology, phagocytosis, and metabolic shifts. We compare the origin and role in neuroinflammation of microglia and monocytes/macrophages. We review potential therapeutic targets to shift microglial polarization. Finally, we review the effect of cell therapy on microglia.
Expert Opinion:
Dysregulated microglial activation after neurologic injury, such as traumatic brain injury, can worsen tissue damage and functional outcomes. There are potential targets in microglia to attenuate this activation, such as proteins and molecules that regulate microglia polarization. Cellular therapeutics that limit, but do not eliminate, the inflammatory response have improved outcomes in animal models by reducing pro-inflammatory microglial activation via secondary signaling. These findings have been replicated in early phase clinical trials.
1. Introduction: Microglia in the CNS And Neurologic Injury
Microglia are the resident tissue macrophages of the central nervous system. They are embryologically distinct from the monocyte/macrophage system in that they are not true myeloid lineage cells, rather they are derived from the primitive yolk sac during development [1]. Nevertheless, they share similarities with the monocyte/macrophage system, with overlapping roles and identities that make characterization of the inflammatory response challenging. Microglia function as danger surveillance cells, and respond via an upregulation of metabolic machinery that enables phagocytosis, release of pro-inflammatory cytokines, and production of enzymes and reactive oxygen species. As with most inflammatory systems, counter-regulatory elements dampen the response and allow transition of activated microglia into reparative cells that enhance tissue repair and remodeling. Here, we review the potential for targeting microglia as a potential avenue for mitigating the secondary damage due to dysregulated inflammation after an injury. The ability to measure this response has developed into a clinically applicable tool as the use of agents to modulate the response becomes more promising. While the literature reviewed is relevant to many disease processes, this review is focused on traumatic injury to the CNS.
Traumatic brain injury (TBI) can be divided into two components: primary and secondary injury. Primary injury is the direct transfer of kinetic energy that leads to tissue disruption and organ dysfunction. Secondary injury is the subsequent development of neuronal excitoxicity, mitochondrial dysfunction, and a hyperinflammatory response [2]. Dysregulated and persistent microglial activation amplifies and propagates the secondary injury of traumatic brain injury. The degree and duration of pro-inflammatory microglial activation correlates with neuronal loss and poor neurocognitive performance in animal models and humans. Loane, et al. demonstrated long term microglial activation after TBI, using a controlled cortical impact (CCI) injury model in mice [3]. They demonstrated that there is an early peak in microglial activation, followed by a prolonged activation detectable at one-year post-injury. Activated microglia co-localize to regions in which there is progressive white matter volumetric loss; in their model, they demonstrated a loss in corpus callosum volume and decline in myelin basic protein, indicating de-myelination as a mechanism of decline. Numerous other investigators have demonstrated microglial activation using simple cell surface markers, but as discussed below, there are potential pitfalls in distinguishing infiltrating monocyte/macrophages from resident tissue microglia. This is potentially important, as there may be discrete differences in function when evaluating these cell types. The reason that persistent activation may be problematic is related to correlative studies in humans. With the advent of more advanced neuroimaging using metabolic positron emission tomography (PET), linked to magnetic resonance imaging (MRI), the degree of microglial metabolic activity can be quantified and repetitively measured. Persistent activation correlates with pain syndromes and many post-TBI symptoms reported by patients after injury [4, 5]. Persistent microglial activation localizes to the thalamus and other mid-brain structures in rodent models and humans [6]. It has been hypothesized that this represents axonal die back or Wallerian degeneration (the degeneration of axons and myelin distal to nerve injury site [7], of cortical projections from the mid-brain).
Activation without other phenotypic or functional assay assessment can be potentially misleading when attempting to determine if the inflammatory response is beneficial or injurious. In most animal and human models, it has been reasonably established that some moderate degree of inflammation is required to initiate wound healing, manage damaged tissues, and prevent uncontrolled infection. However, a dysregulated and persistent or hyper-inflammatory response to injury has off-target effects on un-injured tissue and organs, which contributes to organ failure. Some degree of microglial activity and signaling is beneficial post-injury. Depletion studies often demonstrate amplification of the injury relative to non-depleted controls [8]. Persistent activation is principally pro-inflammatory and not reparative after TBI, and the use of the imaging of the translocator protein TSPO on activated microglia has been shown to be selective for pro-inflammatory polarized astrocytes and microglia, and not anti-inflammatory or reparative cells [9].
Our global hypothesis has been that diminishing the pro-inflammatory response without eliminating it entirely can mitigate consequences and severity of secondary TBI. To effectively target microglial activation and reliably test this hypothesis, a number of other data sets are required: (1) Robust microglial phenotyping in model systems, (2) Functional assays of microglial activity and metabolism, (3) Translationally relevant markers of microglial activity, (4) Distinction between resident microglia and infiltrating monocytes/microglia, (5) Identification of druggable targets and signal transduction cascades that influence microglial behavior.
2. Literature Search Methods
Articles for this review were found using Pubmed.gov through the National Institutes of Health. The primary keywords used in initial searches were traumatic brain injury, neuroinflammation, and microglia. Searches were narrowed to provide evidence on the features of microglia, including activation, polarization, metabolism, and phenotype markers. The searches were also narrowed to identify and review studies that utilized PET-imaging to study microglial activation. Our search for PET-imaging studies also considered those that used particular radioligands to identify microglia. For the cell therapy section, the literature search was narrowed to include only articles showing use of cell therapy in the setting of TBI and neuroinflammation. The studies used for the discussion in this review include clinical studies of human patients afflicted by neuroinflammatory conditions, and preclinical studies with in vivo animal or in vitro cell models of neurologic disease.
3. Immunophenotyping of Microglia
Microglia are most frequently identified and described using immunostaining techniques coupled with either immunohistochemistry (IHC) or flow cytometry. The most frequently used markers are generally shared with other myeloid lineage cells, including monocytes and macrophages. For many studies, common myeloid markers like CD11b/c [10] and ionized calcium-binding adapter molecule 1 (Iba1) [11] are generally sufficient to distinguish rat microglia from peripheral monocytes and macrophages, which are relatively scarce in nervous tissue.
Infiltrating monocytes and CNS-associated macrophages can confound studies, as both contribute to neuroinflammation in injury and disease [12]. Peripheral monocytes and macrophages cross the blood-brain barrier (BBB) and contribute to the local neuroinflammatory response. In circumstances where both invasive myeloid cells and microglia are present, there are a few markers that can vary between species. Mice microglia can be identified by expression of C-X3-C motif chemokine receptor 1 (Cx3cr1) [13, 14]. Other myeloid populations can also express Cx3cr1 [15, 16].
Transcriptome analysis for unique, highly expressed markers identified mouse and human transmembrane protein 119 (TMEM119) and human P2Y12 as candidate markers unique to microglia [17, 18]. However, key limitations remain that reduce the usefulness of TMEM119 and P2Y12 in the setting of injury and disease. Microglia proliferate in response to tissue damage and TMEM119 is not expressed in immature proliferating microglia [17]. Similarly, activated microglia have reduced expression of P2Y12 [18–20]. Recent studies have found that sialic acid binding immunoglobulin-like lectin H (Siglec-H) may be useful in distinguishing mouse and human microglia from other myeloid cell populations using molecular techniques [21–23], flow cytometry [24], and IHC [25].
New tools and analysis use technological advances to identify and better understand microglial phenotypes and biology. Single cell RNA sequencing (scRNAseq) is now capable of identifying and characterizing microglia based upon each cell’s individual transcriptome [26–29]. While powerful, scRNAseq is expensive and captures a limited sample size (approximately 2,000 cells). Multicolor flow cytometry and mass cytometry is used by our group and others. Flow and mass cytometry uses combinations of markers and relative intensity of staining to identify and characterize changes in mouse, rat, and human microglial activation and phenotypes [29–33]. Both of these techniques frequently incorporate machine learning algorithms to assist in the analysis of the large and complex data sets. Tools like t-distributed stochastic neighbor embedding (t-SNE) or uniform manifold approximation and projection (UMAP) are used for dimensional reduction [30, 34–36] while unsupervised cluster identification is assisted by spanning-tree progression analysis of density-normalized events (SPADE), FlowSOM, Phenograph and others [37–39]. The growing use of machine learning and other unsupervised algorithms has the added benefit of substantially reducing potential bias introduced during manual data handling steps [40].
4. Microglial Functional Assays
Microglia survey the nervous system for any inflammatory insult and respond to that insult, acting as the resident immune cell of the CNS. In TBI for example, microglial response occurs during the secondary injury phase, which is marked by a hyperinflammatory response. Historically, microglia activation has been characterized in a binary system: a pro-inflammatory state (M1; Figure 1) versus an anti-inflammatory state (M2; Figure 2). This perspective is shifting to one of activated microglia having a mixed phenotype in response to stimuli, and “resting” microglia actively surveying their cerebral microenvironment. Still, there appears to be some truth to activation of microglia by an inflammatory insult inducing changes in the functionality of microglia. For the purposes of this review, we will discuss microglia with reference to the classic M1 and M2 phenotypes.
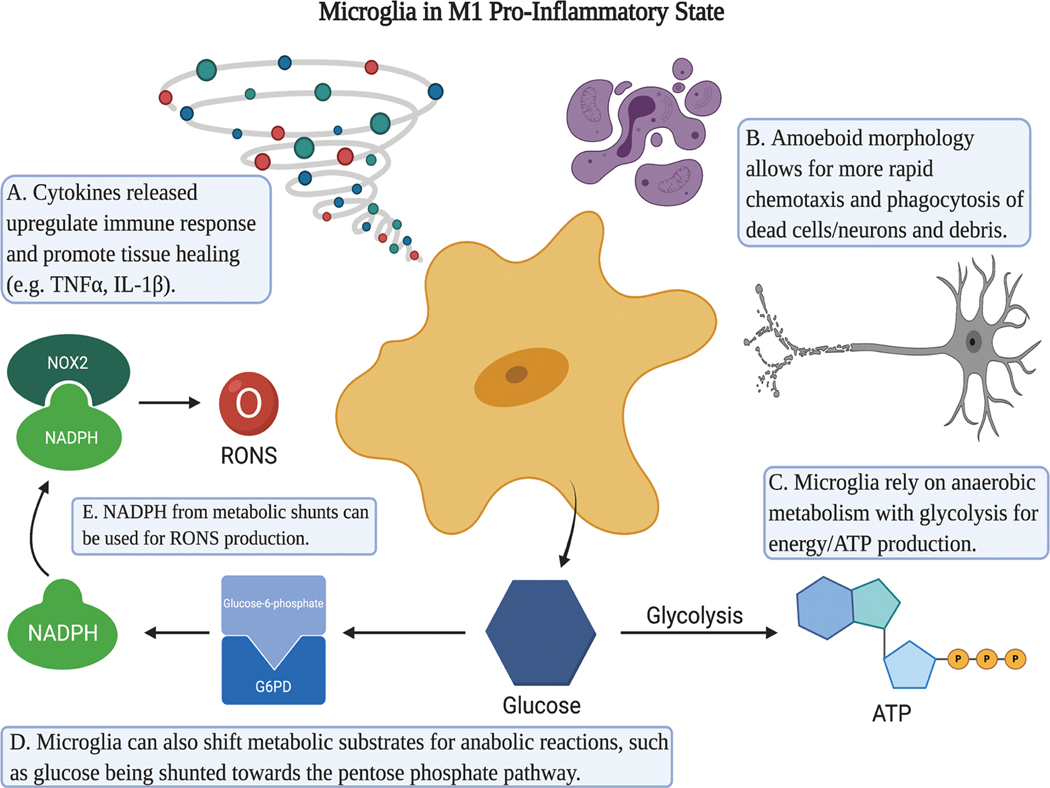
Figure 1.
Microglia in the M1 Pro-Inflammatory State. M1 Microglia release pro-inflammatory cytokines such as TNF-α and IL-1β that propagate the pro-inflammatory response of itself and other microglia or inflammatory cells (A). They typically conform to an amoeboid morphology to allow for rapid chemotaxis and phagocytosis of cellular debris (B). In utilization of glucose, M1 microglia convert glucose to ATP using anaerobic metabolism (C), but can also shunt it (D). Shunts such as the pentose phosphate pathway can increase production of NADPH (E) and thus reactive oxygen and nitrogen species (RONS). Created with BioRender.com.
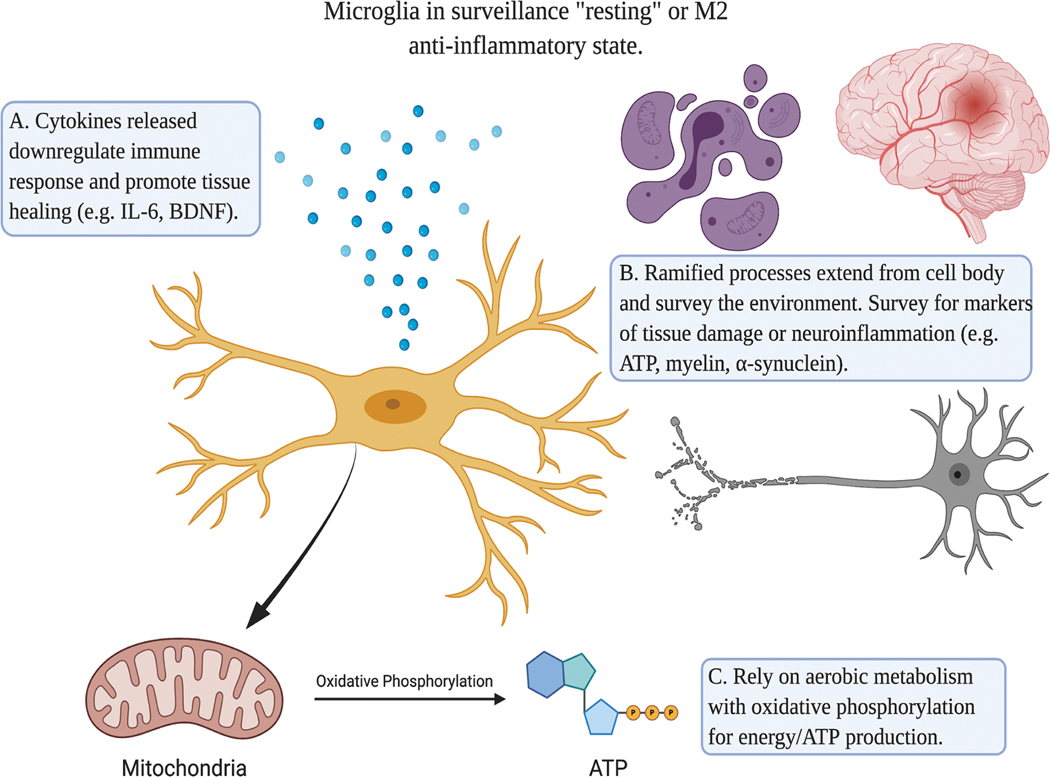
Figure 2.
Microglia in the M2 anti-inflammatory state. M2 microglia release cytokines that downregulate the inflammatory response and promote tissue repair (A). Their morphology consists of ramified processes that reach out and survey their microenvironment for any tissue damage or inflammatory insult (B). These arms are mobile, as they constantly retract and extend. For energy production, anti-inflammatory and resting microglia rely on oxidative phosphorylation for ATP production (C). Created with BioRender.com.
4.1. Cytokines
Microglia secrete cytokines in response to certain stimuli, such as injury. These cytokines stimulate other immune cells, but also stimulate surrounding microglia. Cytokines can be released either freely or via vesicles, which also contain other particles such as micro RNA (miRNA) [41]. If injury induces the activation of the M1 phenotype, microglia secrete pro-inflammatory cytokines, such as interleukin-1 (IL-1), tumor necrosis factor alpha (TNF-α), and interferon gamma (IFN-γ) (Figure 1A). Release of these cytokines can have a multitude of effects. For example, TNF-α can induce cell death in neurons and microglia through its receptor complex [42]. In a rat model of paclitaxel-associated pain, microglial release of interleukin 1 beta (IL-1β) increases astrocyte calcium and neuronal glutamate transporter activity, altering CNS cell signaling [43]. Microglia also release other molecules that stimulate more microglia, increasing their immune response. One example is galectin-3, which has been recognized as a damage molecular pattern associated with multiple disease models [41]. Through an interaction with toll-like receptor 4 (TLR4), galectin-3 from microglia can stimulate the M1 phenotype of other microglia in vitro. Inhibition of this interaction can decrease microglial activation [44].
Some studies have indicated that inhibiting cytokine expression and activity in microglia can decrease the damage caused by microglia. For example, Chio et al. studied treating rodents sustaining a TBI with etanercept, a known TNF-α inhibitor. Etanercept treatment decreased the expression of TNF-α by microglia. They also found that rats treated with etanercept had a decrease in the size of cerebral ischemic injury [45].
Neurologic injury can also induce the M2-phenotype. In the M2 state, microglia typically release anti-inflammatory cytokines to promote healing (Figure 2A). For example, interleukin-6 (IL-6) can promote microglial proliferation. Knockout of IL-6 can impair recovery, through factors such as an increase in oxidative stress and impaired glial activation [46]. IL-6 secretion can increase in response to activation of adenosine receptors, and this effect is potentiated when murine microglia are subjected to a hypoxic environment in vitro [47]. Brain derived neurotrophic factor (BDNF) is a neurotrophin that microglia can release, which exerts a paracrine effect on neighboring neurons. BDNF can alter synaptic development and plasticity of these neighboring neurons [48].
4.2. Free radical formation
In response to an inflammatory insult, microglia can produce reactive oxygen and nitrogen species (RONS) that cause cell damage from oxidative stress, such as lipid membrane peroxidation and DNA damage (Figure 1E). One of the more important enzymes for RONS production in microglia is NADPH oxidase isoform 2 (NOX2). NOX2 is upregulated in the CNS cell population after neurologic injury. After injury, there appears to be a biphasic response in NOX2 activity and expression. There is an initial increase in enzyme activity and expression hours after injury credited to neurons, and a second increase induced by activated microglia. NOX2 appears to play some role in microglia activation; knockout of NOX2 led to a decreased expression of M1 signature genes but an increase of M2 genes. Oxidative stress and cell damage caused by NOX2 may be preventable. Pharmacologic enzyme inhibition with apocynin and knockout NOX2-related genes in mice can decrease neurologic lesion size, extent of neurodegeneration, and markers of cell damage from oxidative stress [49–51]. The upregulation in NOX2 has implications in microglia metabolism, as discussed below.
Peroxynitrite (PN) is a RONS produced by microglia that can lead to cell death and apoptosis. PN is one of the by-products of NOX2 metabolism. Dohi et al. observed elevated PN in the peri-contusion area of mice sustaining cerebral fluid-percussion injury. On immunofluorescent staining, PN co-localized with activated mouse microglia. In this model, knockout of a subunit of NOX2 led to decreased PN staining [51]. While in higher doses it can be lethal for cells, PN at sublethal doses can induce targeting of neurons for phagocytosis. Neher et al. studied rat microglial phagocytosis, and found that PN promoted neuronal expression of phosphatidylserine (PS). PS is a cytoplasmic membrane marker for phagocyte targeting. Breakdown and inhibition of PN production led to decreased PS expression, and subsequently decreased phagocytosis of neurons [52].
An imbalance in antioxidant activity of microglia can also result in oxidative stress. Superoxide dismutase (SOD1) is a cytosolic enzyme that scavenges for RONS [53]. SOD1 activity has been implicated in the development of amyotrophic lateral sclerosis (ALS); this link between microglial SOD1 and ALS has been replicated in a mouse model. The over-expression of microglial SOD1 in transgenic mice can cause motor-neuron death and increased production of RONS [54]. In another mouse model of ALS, mice expressing a mutant human SOD1 were studied. Mouse microglia with this SOD1 mutation expressed higher levels of enzyme; microglia with this mutation were more neurotoxic compared to the wild [55].
4.3. Morphology and Phagocytosis
Microglia typically have two different morphological forms related to their mobility: ramified (Figure 2) and amoeboid (Figure 1) [56]. In the ramified form, the microglia are typically in a surveillance state. Their processes are in direct communication with neighboring neurons and astrocytes. When surveying the CNS, the cellular processes extend out in thin strands in multiple directions. High concentrations of filamentous actin give microglial processes flexibility [57]. Upon encountering a certain injury or stimulus, the microglia can move their process towards the lesion or site of stimulation. When these processes encounter a lesion site, cell signaling then induces microglial migration. That stimulus leads to microglial cell ruffling and a shift towards an amoeboid morphology, allowing the previously stationary cell body to move towards the lesion site [58].
Chemotaxis of microglia to a lesion is influenced by markers of cell damage and neurodegeneration. A common marker of cell damage is adenosine triphosphate (ATP) and its counterpart adenosine diphosphate (ADP). The purinergic P2Y12 receptor on microglia can stimulate this migration in response to ADP or ATP released from damaged cells. Purinergic signaling leads to multiple downstream effects that induce this microglial migration, such as changes in membrane voltage, intracellular calcium concentration, and activation of G-protein coupled receptors [58]. Activation of mouse and rat microglia can increase the expression of the P2Y12 receptor [59, 60]. Inhibition or knockout of the P2Y12 receptor in mice can inhibit microglial activation and migration [60]. Neurodegeneration markers provide a migratory signal for microglia as well. In mouse models of Alzheimer’s disease, activated microglia localize to sites of beta-amyloid plaque aggregates [61].
Microglia in the amoeboid morphology have a larger cell body and are typically responsible for phagocytosis. After migration and chemotaxis towards a chemoattractant, microglia recognize cell-surface markers on cells such as neurons that stimulate the process of phagocytosis. Toll-like receptors (TLRs) and triggering receptor expressed on myeloid cells 2 (TREM2) are two of the primary receptor types involved in signaling microglia for phagocytosis [62] The effects of these receptors have been demonstrated in human and animal models, particularly rats and mice. In relation to neurodegenerative disease, TREM2 receptors may be of higher importance; they recognize and stimulate a response towards apoptotic cellular debris [62]. Takahashi et al. showed the influence TREM2 has on microglia phagocytosis in mice; TREM2 activation led to microglia chemotaxis, cytoskeleton reorganization, and phagocytosis of apoptotic neurons. Knockdown of the TREM2 gene led to decreased phagocytic activity [63]. Phosphatidylserine is an example of these “eat-me” markers found on neurons. Microglia use a glycoprotein, lactadherin, to bind to PS. This forms a bridge between their cell body and the target cell, inducing phagocytosis. Blocking the formation of this bridge between the two cells can inhibit phagocytosis; inhibition of phagocytosis in this manner has also been shown to decrease neuronal cell loss [52]. Other phagocytic signals for microglia can be disease specific, such as alpha-synuclein for Parkinson’s Disease, beta-amyloid plaques for Alzheimer’s disease, and damaged myelin in spinal cord injury or traumatic brain injury [62]. Phagocytosis of myelin in mice appears to be regulated in microglia activation of phosphoinositide 3-kinases (PI3K) by galectin-3 [64].
Phagocytosis by activated microglia may have different functions in the pro- versus anti-inflammatory phenotypes, but it does appear to contribute to tissue regeneration and repair (Figure 1B). M2 anti-inflammatory microglia can use phagocytosis to inhibit the detrimental effects of other inflammatory cells. In a rat model of ischemic stroke, Neumann et al. demonstrated that rat microglia can phagocytose neutrophils and polymorphonuclear cells (PMNs) that invade the stroke area. This decreased the degree of inflammation and neuronal cell damage. Inhibition of microglial phagocytosis led to an increase in neuronal damage and a decrease in neuronal cell viability [65]. Phagocytosis is important for recovery as well; clearance of degenerated myelin may contribute to accelerated myelin regeneration [62]
4.4. Immunometabolism
Recent evidence has emerged regarding the importance of the metabolic pathways and energy production of microglia. Microglia prefer oxidative phosphorylation (OXPHOS) through their mitochondria when acting in the M2 surveillance state (Figure 2C) [66, 67], but will shift to primarily glycolysis in the M1 activated state (Figure 1C–E). Microglia are a versatile cell population, with the ability to utilize different substrates for energy production. In vitro studies have shown that availability of substrates influence how and the rate at which energy is produced. Nagy et al used BV-2 cells, an immortalized murine cell line of microglia, to study these metabolic changes in vitro. After a period of starvation, administration of glucose increased microglial glycolysis. Mitochondrial respiration of microglia decreases in this scenario as well. BV-2 microglia can use glucose, glutamine, beta-hydroxybutyrate, pyruvate, and lactate as substrates for ATP production [68].
In order to mount a response towards injury, microglia induce changes in their metabolic profile. In their surveillance or M2 state, microglia rely more on OXPHOS for ATP production. When activated into the M1 inflammatory state, microglia rely more on anaerobic metabolism [69]. This change to anaerobic metabolism serves two purposes. First, it provides a ready source of energy through glycolysis. Second, it allows microglia to utilize substrates for other pathways necessary for the inflammatory response, such as the pentose-phosphate pathway (PPP).
When stimulated by an inflammatory insult, microglia use anaerobic metabolism, in particular glycolysis, for rapid energy production. Gimeno-Bayón et al. performed in vitro analysis of BV-2 microglia by inducing the M1 inflammatory state with lipopolysaccharides (LPS) and IFN-γ. After stimulation, the BV-2 cells increased glucose consumption, hexokinase activity, and lactate production. While there was no change in the inner membrane potential of mitochondria, the glucose:lactate ratio increased, indicating a shift to anaerobic metabolism by BV-2 microglia [70]. Holland et al obtained similar results in mice when stimulating microglia with IFN-γ; they also demonstrated that expression of the isozyme 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase (PFKB3) increased in microglia after IFN-γ stimulation. PFKB3 is an important allosteric activator of phosphofructokinase-1, the rate-limiting enzyme of glycolysis [71]. To cope with the increased glucose demand, BV-2 microglia will increase expression of glucose-uptake membrane transporters (GLUT), in particular GLUT1 and GLUT4 [70, 72]. Microglia increase GLUT expression in response to increased glucose requirements in the M1 state. Inhibition of glycolysis can attenuate the pro-inflammatory response of stimulated microglia. In vitro studies have demonstrated this with 2-deoxy-D-glucose (2-DG), a glycolytic inhibitor. 2-DG treatment decreases microglial secretion of certain inflammatory cytokines in mice, like TNF-α, IL-1β, and IL-6 [73, 74]
Anaerobic metabolism of microglia allows microglia to utilize glycolytic substrates for other anabolic reactions and pathways. For example, metabolic intermediates can be shunted towards the pentose phosphate pathway (PPP), increasing the production of nicotinamide adenine dinucleotide phosphate (NADPH). NADPH is a product of the PPP. It is used for the production of RONS by NOX2. Glucose-6-phosphate dehydrogenase (G6PD) is the rate-limiting enzyme of the PPP. In vitro and in vivo models of neuroinflammation have shown that activated microglia increase expression and activity of G6PD in BV-2 and mouse microglia [70, 75]. G6PD upregulation is seen primarily in the M1 pro-inflammatory state of microglia, and not in the M2 anti-inflammatory state. Tu et al. demonstrated this using a murine Parkinson’s model. They studied G6PD, the rate-limiting enzyme of the PPP. The activity and expression of G6PD increased in the Parkinson’s model. Rat glial cultures modeling Parkinson’s had an increase in the activity and expression of G6PD. This upregulation correlated with excess production of NADPH and RONS and an increase in dopaminergic neurodegeneration. Pharmacologic inhibition and gene knockout of G6PD decreased dopaminergic neurodegeneration [75].
5. Translationally relevant markers of activation
Markers of microglial activation could provide useful diagnostic tools in the clinical setting (Table 3). TSPO is a commonly studied markers of human microglial activation. TSPO is a transmembrane domain protein found on the outer membrane of mitochondria that was initially discovered as a benzodiazepine-receptor [76]. It is widely distributed in cells throughout the human body. In the CNS, TSPO is primarily localized to astrocytes and microglia [76–78]. The primary function of TSPO remains relatively unclear, but it appears to participate in steroidogenesis, attenuate oxidative stress, regulate apoptosis, and alter cell metabolism [77].
Table 3.
Activations makers of microglia used in diagnostic studies such as PET-imaging.
| Activation Marker | Name/Description | Role in Neuroinflammation | Radioligands |
|---|---|---|---|
| TSPO | Translocator Protein - found on outer membrane of mitochondria | Increased expression in Ml microglia. Possibly involved in steroidogenesis, attenuating oxidative stress, apoptosis, cell metabolism. | PK11195, [11C]PBR28 |
| CB2 | Cannabinoid receptor-2 | Associated with neuronal loss and microglial activation. | [11C]A836339, [11C]NE40 |
| COX-1, COX-2 | Cyclooxygenase | COX-1 - Pro-inflammatory microglia COX-2 - Anti-inflammatory microglia; may attenuate BBB permeability | 11C-ketoprofen methylester |
| P2X7 | Ionotropic purinergic receptor | Upregulated in neurodegeneration. Most responsive to ATP. | 11C-JNJ-54173717 |
| P2Y12 | Metabotropic purinergic receptor | Present only on microglia. Initiates signaling cascades. | None currently available. |
In response to CNS insult or injury, microglia increase the expression of TSPO. This has been demonstrated in multiple neurologic conditions such as ischemic stroke, Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis, multiple sclerosis, and TBI. The degree and localization of TSPO expression is also likely related to the specific condition. Changes in TSPO expression have correlated with the degree of neurologic injury [78]. This increase in TSPO expression has also been demonstrated with in vitro studies of neuroinflammation [79]. In vitro studies of mouse microglia cultures have shown that TSPO expression is more frequently associated with M1 pro-inflammatory microglia as opposed to the M2 anti-inflammatory phenotype [80].
TSPO expression can be observed in PET imaging with the use of specific radioligands. PET-imaging can correlate microglial activation to symptoms of the neurologic disease and injury. PK11195 (PK) was one of the first PET-radioligands developed that has a high affinity for TSPO [81]. In most neurologic diseases studied, PK-binding increases in areas of injury containing a higher density of activated microglia. The distribution of PK-binding varies across pathology and appears to be related to the migration of microglia to the site of injury [78]. This has been demonstrated in human studies. For example, in early-stage Parkinson’s disease, TSPO is observed in higher levels in the striatum. In ALS patients, PK-binding has been observed to increase in the motor cortex, pons, thalamus, and dorsolateral prefrontal cortex, and this increase has been shown to positively correlate with upper motor neuron symptoms [81, 82]. In multiple sclerosis patients, Nutma et al. demonstrated that PK-binding doubled in active and tripled in chronic white matter lesions [83]. In patients with mild cognitive impairment, a presumed precursor to Alzheimer’s disease, Okello et al. showed in PET-imaging that there was a significant increase in PK-binding in areas with increased amyloid-β deposition [84]. Another TSPO ligand that can be used in PET-imaging is [11C]PBR28 (PBR). PBR may have a higher specificity towards TSPO, with up to 90% specific binding in nonhuman primates. In an LPS-induced neuroinflammation model with nonhuman primates, PBR accumulation and distribution increases in the brain. These increases in PBR signal correlate with microglial activation identified on IHC and with increases in IL-1β levels after LPS administration [85, 86].
Targeting TSPO does have some limitations. For example, PK as a ligand has a poor signal-to-noise ratio, a high level of nonspecific binding, a short half-life, and low brain bioavailability [87]. Single gene polymorphisms of TSPO also affect the binding of PK and other potential radioligands. Milenkovic et al. found on computational analysis that the A147T polymorphism can affect TSPO protein structure, stability, and binding; this variant is present in the human population at a frequency of about 30% [88]. Despite its specific binding, PBR has different levels of binding affinities with TSPO based on a single gene polymorphism. This has prompted classification of subjects into low-affinity, high-affinity, and mixed-affinity binding of PBR with TSPO. This variability in affinity creates difficulty in quantifying TSPO density with PET-imaging using PBR as a ligand [81, 89, 90]. Limitations in TSPO with PET imaging prompted the search for other markers of microglial activation and corresponding radioligands.
Cannabinoid receptor-2 (CB2), a G-protein couple receptor, is one potential target. In a mouse model of Alzheimer’s disease (AD), Savonenko et al demonstrated that microglia have increased expression of CB2, with co-localization with microglial marker CD68 in IHC. Using a CB2-specific radiotracer, [11C]A836339, transgenic AD mice showed an increased accumulation of radioactivity compared to controls in whole brain, cortex, and cerebellum. However, given CB2’s presence in neurons, neuronal loss may play some role in quantifying the signal produced. Neuronal loss was not studied in some of these experiments [91, 92]. The only CB2 radiotracer studied in humans to date is [11C]NE40. Favorable uptake of the tracer was detected in healthy control patients, but was found to be decreased in AD patients. Ahmad et al argue that this finding may be due to inherent neuronal loss in Alzheimer’s neurodegeneration, but that uptake of [11C]NE40 is likely due to increased CB2 expression in activated microglia [93].
Cyclooxygenase (COX) isoforms, in particular COX-1 and COX-2, have been studied as potential markers. Both isoforms have been studied in the human and rodent CNS. COX-1 expression appears most relevant as its expression is primarily localized to microglia based on IHC of human cell culture [94], and genetic knockout or inhibition of COX-1 may attenuate the pro-inflammatory microglia in mice [95, 96]. In a rat model of neuroinflammation induced by nigrostriatal injections of either LPS or quinolinic acid, Shukuri et al. showed that 11C-ketoprofen methyl ester can serve as a radioligand for COX-1. PET imaging showed accumulation of 11C-ketoprofen methyl ester in activated microglia after LPS or quinolinic acid induced activation. This correlated with PK-binding of TSPO and IHC staining of activated microglia [97]. COX-2 has some relevance as well, as it may attenuate neuroinflammation and reduce blood-brain barrier (BBB) permeability [96, 98]. Current radioligands for COX isoforms are limited by high non-specific binding for specific isoforms and limited uptake in the target organ [91].
Central to microglial activation are purinergic receptors, the ionotropic P2X7 and metabotropic P2Y12 receptors. P2X7 is upregulated in neurodegenerative conditions. Of purinergic receptors, P2X7 has the lowest affinity to ATP, making it most susceptible to changes in extracellular ATP concentration [99]. P2X7 contributes to pro-inflammatory activation of mouse microglia by stimulating the release of IL-1β [100]. Ory et al. showed that the radiotracer 11C-JNJ-54173717 can cross the blood brain barrier. This radiotracer bonded to humanized P2X7 receptors in rats and monkeys [101]. Most studies related to P2X7 are preclinical, with limited evidence in human models. Janssen et al. found that P2X7 antagonist [11C]SMW139 showed good uptake and visualization of in vivo PET imaging in rats, however, [11C]SMW139 binding was not found to be significant in post-mortem brain tissue of Alzheimer’s disease patients [102]. Alternatively, P2Y12 is present on microglia only [84]. In mice, P2Y12 initiates secondary signaling cascades via PI3K, Protein Kinase A (PKA), Phospholipase C (PLC) and Extracellular signal-regulated kinases (ERKs). This results in microglia undergoing considerable F-actin polymerization that result in eventual chemotaxis [59, 60]. In an in vitro multiple sclerosis model with rat and human microglia, Beaino et al. found that P2Y12 was downregulated in activated microglia. In contrast, P2X7 in their study was upregulated in these activated microglia [103]. Although a PET marker has not been developed for P2Y12, it would a valuable marker to develop that is specific only to microglia and not astrocytes or infiltrating macrophages.
6. Microglia v. Monocytes/Macrophages
Microglia are not the only phagocytic cell involved in neuroinflammation. Peripheral monocytes and macrophages (M/M) migrate to the site of neurologic injury. In an ischemic stroke model using middle cerebral artery occlusion (MCAO), Kim et al. provided evidence of this peripheral myeloid cell migration. Using flow cytometry, mice in the stroke group were found to have an increase in peripheral M/M in the affected hemisphere. The spleen was found to be the likely source for these cells. Average splenic volume decreased in the stroke group, and splenectomy prior to MCAO led to a decrease in the accumulation of peripheral M/M. There was a gradual increase in bone marrow M/M in the ensuing 7 days after stroke. The authors believe that while the bone marrow may not be an immediate reservoir of monocytes, it increases production of these cells in response to injury [104]. Recruitment of these myeloid cells is partly mediated by interaction of C-C motif chemokine ligand 2 (CCL2) and C-C chemokine receptor type 2 (CCR2). This CCL2-CCR2 axis is a chemokine-receptor signaling pathway [105, 106]. A deficiency of CCR2-signaling can impede the expansion of macrophages at a lesion site [107]. Certain by-products of the neurologic disease may provide a stimulus for peripheral immune cell recruitment. In a murine Parkinson’s disease model, Ramos et al. found that peripheral monocytes with ingested α-synuclein migrated to the brain and spinal cord [108].
While both are phagocytic cells, there are differences between microglia and macrophages. Microglia are derived from yolk sac erythromyeloid precursors. These precursor cells change to yolk sac pre-macrophages, which can migrate to the embryonic brain prior to the development of the BBB. After this migration, these pre-macrophages develop into microglia. Once the BBB has developed, yolk sac pre-macrophages that did not migrate and fetal liver monocytes can migrate and develop into peripheral tissue resident macrophages [1]. Monocytes originate in the bone marrow from the myeloid progenitor cell line [109]. Microglia in the resting state can be distinguished by low levels of CD45 expression, but in the midst of neuroinflammation, CD45 expression increases [109]. Other markers distinguish microglia from peripheral phagocytes, such as P2Y12 and transmembrane protein 119 (TMEM119). Monocytes and macrophages express Ly-6c at varying levels, depending on their polarization; this marker can be used to distinguish peripheral cells having migrated to injury site [104]. Given these structural differences, it is important to be able to distinguish microglia from peripheral monocytes and macrophages [107, 108, 110, 111].
Microglia and M/M may also differ in contributions to neuroinflammation. Zarruk et al. found in the MCAO-stroke model that macrophages were more prominently found in the ischemic lesion core, while microglia surrounded the core in the peri-infarct rim. They also found that the inflammatory profiles of each cell differed as well in cytokine and mRNA expression [112]. Russo et al. found similar results in a mild blunt TBI model with mice. Macrophages in the peri-lesion rim were CD206+, indicating a wound-healing or non-inflammatory phenotype, and macrophages in the lesion core were of the pro-inflammatory phenotype. Specific depletion of the non-classical monocytes led to impaired meningeal angiogenesis after injury. Based on their results, classical monocytes or inflammatory macrophages clear and scavenge dead cells in the injury site. The wound-healing macrophages likely derive from a non-classical monocyte, and contribute to angiogenesis. Non-classical monocytes and their derivatives, wound-healing macrophages, contribute to angiogenesis via clearance of extravascular fibrin and an increased expression of matrix metalloproteinase-2 for extracellular matrix remodeling [113].
However, the effects of these peripheral myeloid cells can be detrimental to the process of neuroinflammation as well. Their depletion can attenuate the neuroinflammatory response. Makinde et al. demonstrated that non-classical monocytes recruit neutrophils to the site of injury. Depletion of non-classical monocytes prior to CCI-injury decreased recruitment of neutrophils; this cell depletion reduced cerebral edema as well [114]. Conversely, our lab has shown the consequences of the depletion of peripheral M/M in blunt TBI. Prior to controlled-cortical impact or sham injury, rats were depleted of M/M with clodronate-liposomes. Pre-injury M/M depletion decreased detectable brain microglia and increased BBB damage and permeability in the CCI group [8]. Morganti et al. found that direct antagonism of CCR2 decreased recruitment of macrophages in the hippocampus after CCI in mice. This decrease in macrophage accumulation correlated with decreased expression of NADPH oxidase and decreased cognitive dysfunction after CCI-injury [106]. These and other results show that when targeting microglia to attenuate detrimental neuroinflammation, other myeloid cells have to be considered.
7. Targets That Induce Polarization
Given their importance in most neuroinflammatory conditions, there has been considerable focus in developing therapies that target the activation of microglia. Ideally, a treatment would act as a M1 activation antagonist, a M2 activation agonist, or some combination of the two. A treatment like this could alter the course of neuroinflammation, by shifting microglia polarization from the M1 phenotype (Table 1) to the M2 phenotype (Table 2). A treatment capable of this could promote injury resolution and prevent ongoing tissue damage from microglia. Preclinical studies have shown promise with specific drugs and ligands that shift the M1/M2 polarization balance [115]. In developing targeted therapies that shift microglial polarization, one must consider the range of different targets available, such as enzymes, cell surface markers, transcription factors, and signaling proteins. All of these affect the signaling cascades that induce either a M1 or M2 microglia.
Table 1.
Potential Targets that typically induce M1 microglial polarization, and potential inhibitors used in certain studies.
| Target | Name | Role in Neuroinflammation | Inhibitors |
|---|---|---|---|
| NOX2 | Nitric oxide synthase isoform-2 | Production of RONS. Activates Ml microglial phenotype. |
Gp91ds-tat |
| nSMase | Neutral Sphingomyelinase | Hydrolysis of sphingomyelin contributes to neurodegeneration. | Altenusin |
| Fibrinogen | Fibrinogen | Acute phase reactant present in BBB disruption. Binds Mac-1 receptor to activate Ml-phenotype. |
γ377–395 |
| HMGB1 | High-mobility group box 1 | Stabilizes nuclear chromatin. Activates Ml-phenotype. | Glycyrrhizin |
| SIP | Sphingosine 1-phosphate | Sphingolipid that acts through GPCRs to activate Ml-phenotype. | SIP Receptor Direct Inhibitor (CAY10444) |
| CysLTR | Cysteinyl leukotriene receptor | Upregulates Ml-phenotype through the NF-kB pathway. | HAMI3379 |
| TLR4 | Toll-like Receptor 4 | Activates Ml-phenotype through MAPK pathway. | Pinocembrin |
Table 2.
Potential Targets that typically induce M2 microglial polarization, and potential inhibitors used in certain studies.
| Target | Name | Role in Neuroinflammation | Activators |
|---|---|---|---|
| cAMP | Cyclic Adenosine Monophosphate | With IL-4, can induce M2-phenotype switch. | Gp91ds-tat |
| PPARγ | Peroxisome proliferator-activated receptor | Transcription factor that potentially induces M2-phenotype | Rosiglitazone |
| TrkB/BDNF Pathway | BDNF - Brain-derived neurotrophic factor | Signaling pathway that shifts induced microglia to M2-phenotype | Minocycline |
| β2-receptors | Beta-2 Adrenergic receptors | Increases expression of M2-activation markers (arginase-1, CXCL4, IL-10). | Salmeterol |
| α2-receptors | Alpha-2 Adrenergic receptors | Suppresses pro-inflammatory ERK pathway. | Dexmedetomidine |
A potential target for therapies could be a specific enzyme involved in microglial activation and function. As discussed, previously, NOX2 is an important enzyme in microglia; it appears predominantly in the M1-phenotype and less so in the M2-phenotype. Kumar et al. used gp91ds-tat, a direct inhibitor of NOX2 in a blunt TBI rat model. In mice treated with gp91ds-tat, expression of M1-markers by microglia decreased, but of M2 markers increased. Treatment with gp91ds-tat also led to decreased oxidative stress-induced DNA damage [116]. Neutral Sphingomyelinase (nSMase) is another class of enzymes involved in neuroinflammation. Through its hydrolysis of sphingomyelin, nSMases contribute to neurodegeneration. Kumar et al. studied altenusin to inhibit nSMase. Treatment with altenusin decreased pro-inflammatory cytokine expression by BV-2 microglia in vitro, including TNF-α and IL-1β. The effects of altenusin were dose-dependent as well. In a blunt TBI rodent model, altenusin treatment reduced expression of M1-activation markers, CD68 and NOX2 [117].
A therapy could target a certain molecule that induces microglial activation. Fibrinogen is an acute phase reactant that can become present after a disruption of the BBB, and it can activate microglia. In mice, fibrinogen binds to Macrophage antigen-1 integrin receptor (Mac-1) to activate the M1 phenotype through Ras homolog family member A (RhoA) and PI3K signaling. Blocking CD11b, the alpha-subunit of Mac-1, with an anti-CD11b antibody decreases fibrinogen-mediated activation of microglia. Adams et al. used a fibrin-derived peptide, γ377−395 as a potential inhibitor of this activation cascade in an autoimmune encephalitis mouse model. They found that γ377−395 resulted in decreased pro-inflammatory activation and phagocytic activity in mouse microglia treated with fibrinogen [118]. High-mobility group box 1 (HMGB1) is another molecule that induces M1 activation. HMGB1 localizes to the nucleus and stabilizes chromatin. When released by injured cells, it acts as a pro-inflammatory molecule, and can influence microglia/macrophage polarization. Gao et al. tested the HMGB1-inhibitor glycyrrhizin (GL) in mice using a blunt TBI model. GL-treated injured mice had decreased expression of M1 pro-inflammatory cytokines and mRNA; the M2 phenotype increased in the TBI+GL group [119].
Therapies may not necessarily be limited to new drugs or ligands, but could be from naturally occurring molecules. Cyclic AMP (cAMP) is one example, which was studied by Ghosh et al. When administered with the anti-inflammatory cytokine interleukin-4 (IL-4), cAMP induced a switch in LPS-stimulated microglia from the M1 to M2 phenotype. In a mouse model of spinal cord injury, Ghosh et al. also showed that administration of cAMP and IL-4 after injury led to enhanced production of arginase-1, a marker of the M2 phenotype. The cAMP/IL-4 treatment also led to decreased ROS production in injured mice [120]. Sphingosine 1 phosphate (S1P) is a sphingolipid that acts primarily through G-protein coupled receptors (GCPRs). S1P signaling has been associated with cerebral ischemia. Gaire et al. directly targeted the S1P signaling axis in a MCAO-model of stroke in mice. Direct S1P-receptor (S1P-R) inhibition in mice reduced the number of amoeboid microglia in vivo. Additionally, treatment with the S1P-R inhibitor reduced mRNA expression of M1 surface markers, primarily CD11b, CD16, CD32, and CD86. Treatment also reduced mRNA expression of TNF-α and IL-1β [121].
DNA transcription is another a target in microglia to shift away from the M1-phenotype and towards the M2-phenotype. Peroxisome proliferator-activated receptor gamma (PPARγ) is a ligand-activated transcription factor that appears to contribute to microglial activation and polarization. PPARγ expression increases after M2-activation of microglia induced by IL-4 in vitro. This is associated with a decrease in TSPO expression by microglia, a marker of M1-activation. In M2-polarized microglia obtained from mice, Zhou et al found that inhibition of TSPO with the inhibitor PK led to enhanced expression of PPARγ and of M2-markers, such as CD206 and arginase-1 (Arg-1), in M2-polarized microglia. However, a TSPO agonist decreased PPARγ expression and decreased the release of trophic factors, such as BDNF and insulin-like growth factor 1 (IGF-1), from M2 microglia [122]. Wen et al. used rosiglitazone, a common diabetes mellitus medication, to test activating the PPARγ pathway. Rosiglitazone treatment has been shown to activate the M2-phenotype in in vitro and in vivo mouse models of blunt TBI. Pro-inflammatory cytokine expression decreased with rosiglitazone treatment. Antagonism of the PPARγ pathway actually led to the opposite effect; mouse microglia exhibited the M1-phenotype with PPARγ antagonism [123]. Interestingly, there is some conflicting data on the role of PPARγ in M2-activation. Ji et al. found that direct inhibition of PPARγ shifted polarization in LPS-stimulated rat microglia to the M2-phenotype. PPARγ inhibition increased mRNA levels of M2-markers but decreased mRNA levels of M1 markers [124].
Enhancing or inhibiting a signaling pathway is another potential target for microglia therapies. Cysteinyl leukotriene receptors (CysLTR) mediate inflammatory responses; their expression is upregulated in cerebral ischemia in primary mouse microglia and BV-2 cell cultures. These receptors induce the pro-inflammatory M1-phenotype, through upregulation of the NF-kB pathway (nuclear factor kappa light chain enhancer of activated B cells). Zhao et al. used a direct inhibitor of CysLTR, HAMI3379, in primary mouse microglia and BV2 microglia cultures stimulated by LPS. CysLTR inhibition reduced multiple markers of M1-activation, including NF-kB expression, phagocytic activity, pro-inflammatory cytokine secretion, and CD86 expression [125]. Pinocembrin, a flavanoid with antioxidant and anti-inflammatory properties, inhibits toll-like receptor 4 (TLR4) signaling in microglia to suppress M1 activation. In an intracranial hemorrhage (ICH) mouse model, Lan et al. found that pinocembrin treatment decreased the proportion of M1 microglia, but M2 microglia were not affected. This contributed to the resolution of neuroinflammation in injured mice; injured mice receiving pinocembrin had a smaller lesion size and decreased pro-inflammatory cytokine expression compared to mice receiving vehicle-treatment [126]. Minocycline is a tetracycline-class antibiotic with a similar benefit. In an ICH rat-model, minocycline treatment induced microglia to shift to the M2-phenotype. These microglia all had markers of the M2 anti-inflammatory phenotype, such as arginase-1 and BDNF expression and ramified processes. Miao et al. found that minocycline induces this shift through the tropomyosin-related kinase receptor type B (TrkB)/BDNF signaling pathway in rat microglia [127]. Both pinocembrin and minocycline are lipophilic, which allows them to cross the BBB.
Some medications may act on the adrenergic-signaling to alter microglial polarization. Microglia are known to express β2-receptors, which can influence their activation and morphology when activated or inhibited [128]. Dexmedetomidine, a sedative and anesthetic with ⍺2-adrenergic activity, induces M2-polarization in rat microglia in vitro. Qiu et al. showed that it likely suppressed the pro-inflammatory extracellular signal-regulated kinase (ERK) pathway. In a spinal cord injury rat model, Gao et al. showed that this polarization to the M2 anti-inflammatory phenotype improved functional outcomes and reduced tissue damage in injured rats [129, 130]. Salmeterol, the β2-agonist used in asthma treatment, can alter microglia polarization through its effect on adrenergic pathways as well. Sharma et al. showed that salmeterol administered to LPS-stimulated BV-2 microglia led to a decrease in M1 cytokine and chemokine expression and ROS production. Salmeterol treatment on LPS-stimulated cells also increased the expression of Arg-1, CXC chemokine ligand 4 (CXCL4), interleukin-10 (IL-10), and M2-activation markers [131].
8. Cell Therapy Mechanism of Action
Cell therapy is an alternative strategy that our lab has focused on for the treatment of neuroinflammation. Cell lines considered for use in the treatment of neuroinflammatory conditions, like TBI, include mesenchymal stromal cells (MSCs), multipotent adult progenitor cells (MAPCs), and bone marrow mononuclear cells (BM-MNCs). All of these cell lines can be obtained from a bone marrow harvest [2]. A meta-analysis of preclinical studies using MSC therapy for experimental TBI showed a significant benefit in neurologic outcomes and a reduction in lesion volume size in injured animals [132].
Cell therapy works via loco-regional effects and through modulation of the innate immune response. These cells do not exhibit their effects by direct engraftment into the lesion site. Rather, they localize to the spleen. Our lab has demonstrated that injection of MAPCs into rats sustaining CCI induced splenocyte proliferation, thus preventing the typical reduction in spleen size. This interaction led to an increase in the production of the anti-inflammatory cytokines, IL-4 and IL-10. The injected MAPCs could also be traced to the spleen with immunofluorescence tracing [133]. From here, the infused cells modulate systemic inflammation to limit further damage at the site of neurological injury [2].
Immunomodulation by cell therapy is partly achieved by decreasing the production of pro-inflammatory cytokines by microglia. Liu et al. showed with BV2 microglia cultures that administration of MSCs decreased the microglial production of TNF-⍺ and IL-1β. They also found that this effect is likely due to an inhibition of TLR4-mediated mitogen-activated protein kinase (MAPK) activation [134]. Galindo et al. have demonstrated similar effects in a TBI rodent model. After injury, MSC cell therapy decreased the serum levels of pro-inflammatory cytokines such as TNF-⍺ and IL-1β in injured rats [135].
Cell therapy can also dictate the polarization of microglia as well. Using a controlled cortical impact TBI rat model, rats were administered MAPC therapy, which was compared to a control group. The MAPC group had a significantly higher M2:M1 ratio of microglia, indicating the polarization to the anti-inflammatory phenotype. This mechanism was determined to be due to soluble factors formed after direct contact of MAPCs with splenocytes. These soluble factors may not only induce the M2 phenotype, but they may also lead to increased apoptosis amongst the M1 microglia in vitro. The increase in M1-cell apoptosis was seen with MAPC and BM-MNC treatment [136, 137]. Another key feature seen in these studies, is the proliferation of T regulatory cells (Tregs). Tregs are an important component of systemic inflammation, as they downregulate overactive responses. MAPC therapy in rats sustaining CCI led to an increase in Treg cells at 24 hours post-injury [136]. This is important, as Tregs can reduce the expression of TNF-⍺ from rat microglia [36].
By decreasing microglial activation, cell therapy could potentially improve long-term functional outcomes in patients who have sustained a TBI. In our TBI rodent model, behavior studies have shown that rats receiving cell therapy may have functional recovery that correlates with decreased microglial activation. In one of our lab’s studies, rats were treated with autologous BM-MNCs within 72 hours of CCI. BM-MNC infusion therapy resulted in an increase in apoptosis of pro-inflammatory microglia, and cognitive improvements [138]. In a similar study, we found that the timing of this infusion may affect functional outcomes as well as activation of microglia. MAPC infusion within 24 hours of injury resulted in improved spatial learning and memory; this correlated with a decrease in activated microglia in the dentate gyrus and hippocampus [138, 139]. We have studied combination therapy of MSC infusion with the β-blocker propranolol. The combination of MSC infusion and propranolol resulted in a significant improvement in spatial learning and memory after CCI [140]. Regulatory type T cells (Tregs) should be considered as another cell type for the treatment of TBI. Our lab has shown benefits of Treg therapy in vivo with TBI. After CCI, rats treated with a single dose of umbilical cord-derived Tregs had improvement in neuroinflammation, including a decrease in microglia in the ipsilateral hemisphere 30 days after injury [36]. Given the use of Tregs in other inflammatory conditions, it may be worthwhile to consider their use to attenuate the inflammatory cascade resulting from TBI.
This evidence in the preclinical setting supported translation of cell therapy to human studies. A phase I clinical trial in children with severe TBI was completed at our institution. No adverse events occurred with infusion of autologous BM-MNCs. Phase II clinical trials of cell therapy for severe TBI in adults and children are currently active [141].
9. Conclusion
In this review, we discussed the impact that the microglial response can have upon neurological injury. While they contribute to injury resolution, an overactive response from microglia can perpetuate further tissue damage which can be detrimental to functional outcomes. Therefore, microglia present a potential target for therapy with the intent of resolving neuroinflammation. In order to develop therapies properly directed towards microglia, it is important to understand multiple facets of their response to injury. Microglia can be identified based on multiple parameters of their immunophenotype, including surface markers and their transcriptome. This allows us to distinguish microglia from other phagocytic cells, such as peripheral macrophages and monocytes. Functional assays provide an assessment of the activity of microglia in expressing either a pro-inflammatory or anti-inflammatory phenotype. These functional assays measure secretome, morphology, phagocytic activity, RONS production, and cell metabolism. Markers of activation provide another method of identifying microglia; these markers have clinical translation, as some are seen on certain imaging studies, such as PET scans. This data is necessary to further develop therapies directed towards the microglia response in neurologic injury.
10. Expert Opinion
Our current knowledge on microglial activation and response to neurologic insult, such as TBI, has evolved substantially over the years to reflect the nuance and complexity of neuroinflammation. Microglia exhibit a spectrum of phenotypes across the classic M1 and M2 activation states. Certain components of their response lend to resolution of neuroinflammation. This makes it more difficult to target a single population of microglia or utilize a simple monotherapy, such as anti-inflammatory steroids. However, some agents and known medications have shown promise in ameliorating the pro-inflammatory response of M1-microglia.
The temporal changes in microglial activation can be difficult to understand. However, it is important to recognize this cell’s balance between the pro-inflammatory and anti-inflammatory phenotypes. It is also important to consider the various responses of microglia to injury for therapeutic interventions not only in TBI, but in other neurologic diseases as well. Understanding the full phenotypic spectrum of microglia allows us to understand the cell’s response to neurologic injury and full contribution to neuroinflammation. This information would provide insight of the true cellular components and machinery that drives this inflammatory response. This understanding can help us develop therapeutics that affect microglial polarization and activity more effectively.
Some therapies have been developed for certain targets already, and they have shown promise in preclinical studies. Certain proteins like enzymes are important components of this machinery. One example is the NOX2 enzyme. As previously discussed, NOX2 contributes to free radical production for respiratory burst, but its activity and expression can alter microglial polarization. Altering cell signaling cascades at certain checkpoints could change microglial phenotype by affecting DNA transcription or protein production and expression. Potential therapeutics could either inhibit or activate certain signaling pathways. And they could target components like transcription factors, such as PPARγ or cell surface receptors, such as TLR4 or CysLTR. By knowing the components that drive polarization shifts in microglia, we can more effectively develop therapies that shift microglia to a phenotype that contributes to injury resolution.
In the setting of TBI, it is important to consider peripheral and systemic influences on microglia. TBI in the real-world is usually associated with other traumatic injuries that can affect systemic inflammatory responses as well. Thus, when studying traumatic brain injury, it is important to distinguish the effect of microglia on neuroinflammation from peripheral inflammatory cells. In the setting of polytrauma, these peripheral inflammatory cells may be responding to other organ injury, but in effect create some collateral damage further afflicting the present neurologic injury. This may call for the need to improve the specificity of methods for identifying microglia.
Further development of imaging markers could provide a substantial advancement in developing therapies directed at microglial activity and the associated neuroinflammation. There are some imaging markers currently in use, such as TSPO, but current markers available can be limited by their specificity for microglia. Some markers have only been used in pre-clinical animal models. Some markers, such as CB2, are present in not only microglia, but in neurons as well. If an imaging marker specific for only human microglia were developed, it would provide another measurement available to not only researchers studying neuroinflammatory diseases, but also to clinicians treating patients afflicted by neurologic injury.
Our view is that dampening the pro-inflammatory M1 response without eliminating it entirely can reduce secondary inflammation-associated damage to neurologic tissue and accelerate injury resolution. Some inflammation is important to resolve neurologic injury and facilitate repair, but a completely dysregulated and persistent inflammatory state can limit tissue repair. This persistent neuroinflammation leads to not only further tissue injury, but poor neurocognitive outcomes in the recovery and rehabilitation stage after neurologic injury. We believe that the microglia cell is a primary driver behind this chronic neuroinflammation. Coalescing the research in this review gives us a global view of the microglia as a component of the neuroinflammatory response distinct from peripheral myeloid populations, which is critical to developing new therapies and treatment for neurologic conditions.
Alongside these targeted therapies, cell therapy presents another strategy for dynamically altering the microglial response to neurologic injury. In our experience studying TBI, cell therapies alter microglia activation and improve short-term biomarkers of injury severity and long-term behavioral outcome measures. Given the potential of cell therapy to resolve overactive immune responses in multiple diseases, we believe that cell therapy can attenuate a hyperinflammatory response to neurologic injury. One of the important mechanisms by which it attenuates this hyperinflammatory response is through the modulation of microglia. Early clinical trials have shown cell therapy is safe in the setting of TBI, and is being further evaluated as a potential treatment. Future studies of cell therapy may seek to determine which specific cell provides the most benefit in the setting of TBI-induced neuroinflammation. One example of this would be exploring the potential of other cells, such as Tregs, or even combined cell therapy, such as MSCs or BM-MNCs with Tregs, to attenuate an overactive cascade of neuroinflammation. Future clinical trials may compare multiple variations of cell therapy based on dosing, cell type, and timing of administration in relation to the initial neurologic injury.
ARTICLE HIGHLIGHTS.
Neuroinflammation secondary to traumatic brain injury can continue years after initial injury, and it is characterized by persistent microglial activation.
Microglia have classically been described as having a phenotype that is either pro-inflammatory (M1) or anti-inflammatory (M2). Promoting the M2 response and attenuating the M1 response may increase the likelihood of injury resolution.
Microglial activation can be observed in positron emission tomography (PET) imaging with radioligands specific for microglial markers.
In animal models, targeted therapy has shown some success in shifting microglial polarization away from the M1-phenotype and towards the M2-phenotype.
Therapeutic targets are varied, but include molecules or proteins that affect microglial activation and signaling. Examples include enzymes like NADPH oxidase isoform 2 (NOX2) and signal transduction proteins like peroxisome proliferator-activated receptor gamma (PPARγ). Naturally occurring molecules like cyclic AMP (cAMP) and fibrinogen have also been implicated as potential targets.
Cell therapy has potential in targeting microglia, and has already shown to alleviate neuroinflammation secondary to traumatic brain injury (TBI).
Acknowledgments
Financial Disclosures
The authors of this review are funded by Athersys, CBR Systems, Hope Bio, Biostage, and the National Institute of General Medical Science of the National Institutes of Health (under award number 2T32GM008792).
References
- 1. Li Q. and Barres BA, Microglia and macrophages in brain homeostasis and disease. Nat Rev Immunol, 2018. 18(4): p. 225–242.29151590 * We found this review to be of importance by providing a description of microglia and macrophages in “resting” conditions in the CNS. It helped develop our discussion in distinguishing between the two cell lines, by describing their ontogeny.
- 2.Cox CS Jr., Cellular therapy for traumatic neurological injury. Pediatr Res, 2018. 83(1–2): p. 325–332. [DOI] [PubMed] [Google Scholar]
- 3.Loane DJ, Kumar A, Stoica BA, et al. , Progressive neurodegeneration after experimental brain trauma: association with chronic microglial activation. J Neuropathol Exp Neurol, 2014. 73(1): p. 14–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramlackhansingh AF, Brooks DJ, Greenwood RJ, et al. , Inflammation after trauma: microglial activation and traumatic brain injury. Ann Neurol, 2011. 70(3): p. 374–83. [DOI] [PubMed] [Google Scholar]
- 5.Prossin AJ,J; Savitz S; Zubieta JK; Dantzer R; Cox CS., Preliminary in vivo evidence of neuroimmune activation in chronic pain states in humans: Analgesic reversal with autologous stem cell treatment. Am Coll Neuropsychopharmacology, 2018. 57(T254). [Google Scholar]
- 6.Raghavendra Rao VL, Dogan A, Bowen KK, et al. , Traumatic brain injury leads to increased expression of peripheral-type benzodiazepine receptors, neuronal death, and activation of astrocytes and microglia in rat thalamus. Exp Neurol, 2000. 161(1): p. 102–14. [DOI] [PubMed] [Google Scholar]
- 7.Rotshenker S, Wallerian degeneration: the innate-immune response to traumatic nerve injury. J Neuroinflammation, 2011. 8: p. 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aertker BM, Kumar A, Prabhakara KS, et al. , Pre-injury monocyte/macrophage depletion results in increased blood-brain barrier permeability after traumatic brain injury. J Neurosci Res, 2019. 97(6): p. 698–707. [DOI] [PubMed] [Google Scholar]
- 9.Pannell M, Economopoulos V, Wilson TC, et al. , Imaging of translocator protein upregulation is selective for pro-inflammatory polarized astrocytes and microglia. Glia, 2020. 68(2): p. 280–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robinson AP, White TM, and Mason DW, Macrophage heterogeneity in the rat as delineated by two monoclonal antibodies MRC OX-41 and MRC OX-42, the latter recognizing complement receptor type 3. Immunology, 1986. 57(2): p. 239–47. [PMC free article] [PubMed] [Google Scholar]
- 11.Ito D, Imai Y, Ohsawa K, et al. , Microglia-specific localisation of a novel calcium binding protein, Iba1. Brain Res Mol Brain Res, 1998. 57(1): p. 1–9. [DOI] [PubMed] [Google Scholar]
- 12.Prinz M, Tay TL, Wolf Y, et al. , Microglia: unique and common features with other tissue macrophages. Acta Neuropathol, 2014. 128(3): p. 319–31. [DOI] [PubMed] [Google Scholar]
- 13.Jung S, Aliberti J, Graemmel P, et al. , Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol, 2000. 20(11): p. 4106–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boillee S, Yamanaka K, Lobsiger CS, et al. , Onset and progression in inherited ALS determined by motor neurons and microglia. Science, 2006. 312(5778): p. 1389–92. [DOI] [PubMed] [Google Scholar]
- 15.Goldmann T, Wieghofer P, Jordao MJ, et al. , Origin, fate and dynamics of macrophages at central nervous system interfaces. Nat Immunol, 2016. 17(7): p. 797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wieghofer P, Knobeloch KP, and Prinz M, Genetic targeting of microglia. Glia, 2015. 63(1): p. 1–22. [DOI] [PubMed] [Google Scholar]
- 17.Bennett ML, Bennett FC, Liddelow SA, et al. , New tools for studying microglia in the mouse and human CNS. Proc Natl Acad Sci U S A, 2016. 113(12): p. E1738–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mildner A, Huang H, Radke J, et al. , P2Y12 receptor is expressed on human microglia under physiological conditions throughout development and is sensitive to neuroinflammatory diseases. Glia, 2017. 65(2): p. 375–387. [DOI] [PubMed] [Google Scholar]
- 19.Amadio S, Parisi C, Montilli C, et al. , P2Y(12) receptor on the verge of a neuroinflammatory breakdown. Mediators Inflamm, 2014. 2014: p. 975849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mildner A, Ghosts in the shell: identification of microglia in the human central nervous system by P2Y12 receptor. Neural Regen Res, 2017. 12(4): p. 570–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bedard A, Tremblay P, Chernomoretz A, et al. , Identification of genes preferentially expressed by microglia and upregulated during cuprizone-induced inflammation. Glia, 2007. 55(8): p. 777–89. [DOI] [PubMed] [Google Scholar]
- 22.Gautier EL, Shay T, Miller J, et al. , Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol, 2012. 13(11): p. 1118–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Butovsky O, Jedrychowski MP, Moore CS, et al. , Identification of a unique TGF-beta-dependent molecular and functional signature in microglia. Nat Neurosci, 2014. 17(1): p. 131–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiu IM, Morimoto ET, Goodarzi H, et al. , A neurodegeneration-specific gene-expression signature of acutely isolated microglia from an amyotrophic lateral sclerosis mouse model. Cell Rep, 2013. 4(2): p. 385–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Konishi H, Kobayashi M, Kunisawa T, et al. , Siglec-H is a microglia-specific marker that discriminates microglia from CNS-associated macrophages and CNS-infiltrating monocytes. Glia, 2017. 65(12): p. 1927–1943. [DOI] [PubMed] [Google Scholar]
- 26.Esaulova E, Cantoni C, Shchukina I, et al. , Single-cell RNA-seq analysis of human CSF microglia and myeloid cells in neuroinflammation. Neurol Neuroimmunol Neuroinflamm, 2020. 7(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hammond TR, Dufort C, Dissing-Olesen L, et al. , Single-Cell RNA Sequencing of Microglia throughout the Mouse Lifespan and in the Injured Brain Reveals Complex Cell-State Changes. Immunity, 2019. 50(1): p. 253–271 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Q, Cheng Z, Zhou L, et al. , Developmental Heterogeneity of Microglia and Brain Myeloid Cells Revealed by Deep Single-Cell RNA Sequencing. Neuron, 2019. 101(2): p. 207–223 e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sousa C, Golebiewska A, Poovathingal SK, et al. , Single-cell transcriptomics reveals distinct inflammation-induced microglia signatures. EMBO Rep, 2018. 19(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Toledano Furman N, Gottlieb A, Prabhakara KS, et al. , High-resolution and differential analysis of rat microglial markers in traumatic brain injury: conventional flow cytometric and bioinformatics analysis. Sci Rep, 2020. 10(1): p. 11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Toledano Furman NE, Prabhakara KS, Bedi S, et al. , OMIP-041: Optimized multicolor immunofluorescence panel rat microglial staining protocol. Cytometry A, 2018. 93(2): p. 182–185. [DOI] [PubMed] [Google Scholar]
- 32.Sankowski R, Bottcher C, Masuda T, et al. , Mapping microglia states in the human brain through the integration of high-dimensional techniques. Nat Neurosci, 2019. 22(12): p. 2098–2110. [DOI] [PubMed] [Google Scholar]
- 33.Dukhinova M, Kopeikina E, and Ponomarev ED, Usage of Multiparameter Flow Cytometry to Study Microglia and Macrophage Heterogeneity in the Central Nervous System During Neuroinflammation and Neurodegeneration. Methods Mol Biol, 2018. 1745: p. 167–177. [DOI] [PubMed] [Google Scholar]
- 34.Amir el AD, Davis KL, Tadmor MD, et al. , viSNE enables visualization of high dimensional single-cell data and reveals phenotypic heterogeneity of leukemia. Nat Biotechnol, 2013. 31(6): p. 545–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Becht E, McInnes L, Healy J, et al. , Dimensionality reduction for visualizing single-cell data using UMAP. Nat Biotechnol, 2018. [DOI] [PubMed] [Google Scholar]
- 36.Caplan HW, Prabhakara KS, Kumar A, et al. , Human cord blood-derived regulatory T-cell therapy modulates the central and peripheral immune response after traumatic brain injury. Stem Cells Transl Med, 2020. 9(8): p. 903–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gautreau G, Pejoski D, Le Grand R, et al. , SPADEVizR: an R package for visualization, analysis and integration of SPADE results. Bioinformatics, 2017. 33(5): p. 779–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Gassen S, Callebaut B, Van Helden MJ, et al. , FlowSOM: Using self-organizing maps for visualization and interpretation of cytometry data. Cytometry A, 2015. 87(7): p. 636–45. [DOI] [PubMed] [Google Scholar]
- 39.Weber LM and Robinson MD, Comparison of clustering methods for high-dimensional single-cell flow and mass cytometry data. Cytometry A, 2016. 89(12): p. 1084–1096. [DOI] [PubMed] [Google Scholar]
- 40.Finak G, Langweiler M, Jaimes M, et al. , Standardizing Flow Cytometry Immunophenotyping Analysis from the Human ImmunoPhenotyping Consortium. Sci Rep, 2016. 6: p. 20686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodriguez-Gomez JA, Kavanagh E, Engskog-Vlachos P, et al. , Microglia: Agents of the CNS Pro-Inflammatory Response. Cells, 2020. 9(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith JA, Das A, Ray SK, et al. , Role of pro-inflammatory cytokines released from microglia in neurodegenerative diseases. Brain Res Bull, 2012. 87(1): p. 10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yan X, Li F, Maixner DW, et al. , Interleukin-1beta released by microglia initiates the enhanced glutamatergic activity in the spinal dorsal horn during paclitaxel-associated acute pain syndrome. Glia, 2019. 67(3): p. 482–497. [DOI] [PubMed] [Google Scholar]
- 44.Burguillos MA, Svensson M, Schulte T, et al. , Microglia-Secreted Galectin-3 Acts as a Toll-like Receptor 4 Ligand and Contributes to Microglial Activation. Cell Rep, 2015. 10(9): p. 1626–1638. [DOI] [PubMed] [Google Scholar]
- 45.Chio CC, Chang CH, Wang CC, et al. , Etanercept attenuates traumatic brain injury in rats by reducing early microglial expression of tumor necrosis factor-alpha. BMC Neurosci, 2013. 14: p. 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Erta M, Quintana A, and Hidalgo J, Interleukin-6, a major cytokine in the central nervous system. Int J Biol Sci, 2012. 8(9): p. 1254–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Merighi S, Bencivenni S, Vincenzi F, et al. , A2B adenosine receptors stimulate IL-6 production in primary murine microglia through p38 MAPK kinase pathway. Pharmacol Res, 2017. 117: p. 9–19. [DOI] [PubMed] [Google Scholar]
- 48.Parkhurst CN, Yang G, Ninan I, et al. , Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell, 2013. 155(7): p. 1596–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang J, Ma MW, Dhandapani KM, et al. , Regulatory role of NADPH oxidase 2 in the polarization dynamics and neurotoxicity of microglia/macrophages after traumatic brain injury. Free Radic Biol Med, 2017. 113: p. 119–131. [DOI] [PubMed] [Google Scholar]
- 50.Zhang QG, Laird MD, Han D, et al. , Critical role of NADPH oxidase in neuronal oxidative damage and microglia activation following traumatic brain injury. PLoS One, 2012. 7(4): p. e34504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dohi K, Ohtaki H, Nakamachi T, et al. , Gp91phox (NOX2) in classically activated microglia exacerbates traumatic brain injury. J Neuroinflammation, 2010. 7: p. 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Neher JJ, Neniskyte U, Zhao JW, et al. , Inhibition of microglial phagocytosis is sufficient to prevent inflammatory neuronal death. J Immunol, 2011. 186(8): p. 4973–83. [DOI] [PubMed] [Google Scholar]
- 53.Mondola P, Damiano S, Sasso A, et al. , The Cu, Zn Superoxide Dismutase: Not Only a Dismutase Enzyme. Front Physiol, 2016. 7: p. 594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xiao Q, Zhao W, Beers DR, et al. , Mutant SOD1(G93A) microglia are more neurotoxic relative to wild-type microglia. J Neurochem, 2007. 102(6): p. 2008–2019. [DOI] [PubMed] [Google Scholar]
- 55.Liao B, Zhao W, Beers DR, et al. , Transformation from a neuroprotective to a neurotoxic microglial phenotype in a mouse model of ALS. Exp Neurol, 2012. 237(1): p. 147–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kettenmann H, Hanisch UK, Noda M, et al. , Physiology of microglia. Physiol Rev, 2011. 91(2): p. 461–553. [DOI] [PubMed] [Google Scholar]
- 57.Capani F, Ellisman MH, and Martone ME, Filamentous actin is concentrated in specific subpopulations of neuronal and glial structures in rat central nervous system. Brain Res, 2001. 923(1–2): p. 1–11. [DOI] [PubMed] [Google Scholar]
- 58.Madry C. and Attwell D, Receptors, ion channels, and signaling mechanisms underlying microglial dynamics. J Biol Chem, 2015. 290(20): p. 12443–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu T, Zhang X, Shi H, et al. , P2Y12 regulates microglia activation and excitatory synaptic transmission in spinal lamina II neurons during neuropathic pain in rodents. Cell Death Dis, 2019. 10(3): p. 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Haynes SE, Hollopeter G, Yang G, et al. , The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat Neurosci, 2006. 9(12): p. 1512–9. [DOI] [PubMed] [Google Scholar]
- 61.Rodriguez JJ, Witton J, Olabarria M, et al. , Increase in the density of resting microglia precedes neuritic plaque formation and microglial activation in a transgenic model of Alzheimer’s disease. Cell Death Dis, 2010. 1: p. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fu R, Shen Q, Xu P, et al. , Phagocytosis of microglia in the central nervous system diseases. Mol Neurobiol, 2014. 49(3): p. 1422–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Takahashi K, Rochford CD, and Neumann H, Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2. J Exp Med, 2005. 201(4): p. 647–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rotshenker S, Reichert F, Gitik M, et al. , Galectin-3/MAC-2, Ras and PI3K activate complement receptor-3 and scavenger receptor-AI/II mediated myelin phagocytosis in microglia. Glia, 2008. 56(15): p. 1607–13. [DOI] [PubMed] [Google Scholar]
- 65.Neumann J, Sauerzweig S, Ronicke R, et al. , Microglia cells protect neurons by direct engulfment of invading neutrophil granulocytes: a new mechanism of CNS immune privilege. J Neurosci, 2008. 28(23): p. 5965–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ghosh S, Castillo E, Frias ES, et al. , Bioenergetic regulation of microglia. Glia, 2018. 66(6): p. 1200–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Harry GJ, Childers G, Giridharan S, et al. , An association between mitochondria and microglia effector function. What do we think we know? Neuroimmunol Neuroinflamm, 2020. 7: p. 150–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nagy AM, Fekete R, Horvath G, et al. , Versatility of microglial bioenergetic machinery under starving conditions. Biochim Biophys Acta Bioenerg, 2018. 1859(3): p. 201–214. [DOI] [PubMed] [Google Scholar]
- 69. Devanney NA, Stewart AN, and Gensel JC, Microglia and macrophage metabolism in CNS injury and disease: The role of immunometabolism in neurodegeneration and neurotrauma. Exp Neurol, 2020. 329: p. 113310. * This review provided information on microglia immunometabolism, which nelped us to consider how metabolic shifts affected microglia polarization.
- 70.Gimeno-Bayon J, Lopez-Lopez A, Rodriguez MJ, et al. , Glucose pathways adaptation supports acquisition of activated microglia phenotype. J Neurosci Res, 2014. 92(6): p. 723–31. [DOI] [PubMed] [Google Scholar]
- 71.Holland R, McIntosh AL, Finucane OM, et al. , Inflammatory microglia are glycolytic and iron retentive and typify the microglia in APP/PS1 mice. Brain Behav Immun, 2018. 68: p. 183–196. [DOI] [PubMed] [Google Scholar]
- 72.Wang L, Pavlou S, Du X, et al. , Glucose transporter 1 critically controls microglial activation through facilitating glycolysis. Mol Neurodegener, 2019. 14(1): p. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fodelianaki G, Lansing F, Bhattarai P, et al. , Nerve Growth Factor modulates LPS - induced microglial glycolysis and inflammatory responses. Exp Cell Res, 2019. 377(1–2): p. 10–16. [DOI] [PubMed] [Google Scholar]
- 74.Wang Q, Zhao Y, Sun M, et al. , 2-Deoxy-d-glucose attenuates sevoflurane-induced neuroinflammation through nuclear factor-kappa B pathway in vitro. Toxicol In Vitro, 2014. 28(7): p. 1183–9. [DOI] [PubMed] [Google Scholar]
- 75.Tu D, Gao Y, Yang R, et al. , The pentose phosphate pathway regulates chronic neuroinflammation and dopaminergic neurodegeneration. J Neuroinflammation, 2019. 16(1): p. 255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Dupont AC, Largeau B, Santiago Ribeiro MJ, et al. , Translocator Protein-18 kDa (TSPO) Positron Emission Tomography (PET) Imaging and Its Clinical Impact in Neurodegenerative Diseases. Int J Mol Sci, 2017. 18(4). * This article reviews not just the role of TSPO in PET-imaging, but also the development of multiple agents to target this specific biomarker in the setting of multiple different CNS disorders.
- 77.Bonsack F. and Sukumari-Ramesh S, TSPO: An Evolutionarily Conserved Protein with Elusive Functions. Int J Mol Sci, 2018. 19(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen MK and Guilarte TR, Translocator protein 18 kDa (TSPO): molecular sensor of brain injury and repair. Pharmacol Ther, 2008. 118(1): p. 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ory D, Planas A, Dresselaers T, et al. , PET imaging of TSPO in a rat model of local neuroinflammation induced by intracerebral injection of lipopolysaccharide. Nucl Med Biol, 2015. 42(10): p. 753–61. [DOI] [PubMed] [Google Scholar]
- 80.Beckers L, Ory D, Geric I, et al. , Increased Expression of Translocator Protein (TSPO) Marks Pro-inflammatory Microglia but Does Not Predict Neurodegeneration. Mol Imaging Biol, 2018. 20(1): p. 94–102. [DOI] [PubMed] [Google Scholar]
- 81.Best L, Ghadery C, Pavese N, et al. , New and Old TSPO PET Radioligands for Imaging Brain Microglial Activation in Neurodegenerative Disease. Curr Neurol Neurosci Rep, 2019. 19(5): p. 24. [DOI] [PubMed] [Google Scholar]
- 82.Turner MR, Cagnin A, Turkheimer FE, et al. , Evidence of widespread cerebral microglial activation in amyotrophic lateral sclerosis: an [11C](R)-PK11195 positron emission tomography study. Neurobiol Dis, 2004. 15(3): p. 601–9. [DOI] [PubMed] [Google Scholar]
- 83.Nutma E, Stephenson JA, Gorter RP, et al. , A quantitative neuropathological assessment of translocator protein expression in multiple sclerosis. Brain, 2019. 142(11): p. 3440–3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Okello A, Edison P, Archer HA, et al. , Microglial activation and amyloid deposition in mild cognitive impairment: a PET study. Neurology, 2009. 72(1): p. 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hillmer AT, Holden D, Fowles K, et al. , Microglial depletion and activation: A [(11)C]PBR28 PET study in nonhuman primates. EJNMMI Res, 2017. 7(1): p. 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hannestad J, Gallezot JD, Schafbauer T, et al. , Endotoxin-induced systemic inflammation activates microglia: [(1)(1)C]PBR28 positron emission tomography in nonhuman primates. Neuroimage, 2012. 63(1): p. 232–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chauveau F, Boutin H, Van Camp N, et al. , Nuclear imaging of neuroinflammation: a comprehensive review of [11C]PK11195 challengers. Eur J Nucl Med Mol Imaging, 2008. 35(12): p. 2304–19. [DOI] [PubMed] [Google Scholar]
- 88.Milenkovic VM, Bader S, Sudria-Lopez D, et al. , Effects of genetic variants in the TSPO gene on protein structure and stability. PLoS One, 2018. 13(4): p. e0195627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Owen DR, Gunn RN, Rabiner EA, et al. , Mixed-affinity binding in humans with 18-kDa translocator protein ligands. J Nucl Med, 2011. 52(1): p. 24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Owen DR, Yeo AJ, Gunn RN, et al. , An 18-kDa translocator protein (TSPO) polymorphism explains differences in binding affinity of the PET radioligand PBR28. J Cereb Blood Flow Metab, 2012. 32(1): p. 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Janssen B, Vugts DJ, Windhorst AD, et al. , PET Imaging of Microglial Activation-Beyond Targeting TSPO. Molecules, 2018. 23(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Savonenko AV, Melnikova T, Wang Y, et al. , Cannabinoid CB2 Receptors in a Mouse Model of Abeta Amyloidosis: Immunohistochemical Analysis and Suitability as a PET Biomarker of Neuroinflammation. PLoS One, 2015. 10(6): p. e0129618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ahmad R, Postnov A, Bormans G, et al. , Decreased in vivo availability of the cannabinoid type 2 receptor in Alzheimer’s disease. Eur J Nucl Med Mol Imaging, 2016. 43(12): p. 2219–2227. [DOI] [PubMed] [Google Scholar]
- 94.Hoozemans JJ, Rozemuller AJ, Janssen I, et al. , Cyclooxygenase expression in microglia and neurons in Alzheimer’s disease and control brain. Acta Neuropathol, 2001. 101(1): p. 2–8. [DOI] [PubMed] [Google Scholar]
- 95.Choi SH, Langenbach R, and Bosetti F, Genetic deletion or pharmacological inhibition of cyclooxygenase-1 attenuate lipopolysaccharide-induced inflammatory response and brain injury. FASEB J, 2008. 22(5): p. 1491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Aid S. and Bosetti F, Targeting cyclooxygenases-1 and −2 in neuroinflammation: Therapeutic implications. Biochimie, 2011. 93(1): p. 46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shukuri M, Takashima-Hirano M, Tokuda K, et al. , In vivo expression of cyclooxygenase-1 in activated microglia and macrophages during neuroinflammation visualized by PET with 11C-ketoprofen methyl ester. J Nucl Med, 2011. 52(7): p. 1094–101. [DOI] [PubMed] [Google Scholar]
- 98.Aid S, Silva AC, Candelario-Jalil E, et al. , Cyclooxygenase-1 and −2 differentially modulate lipopolysaccharide-induced blood-brain barrier disruption through matrix metalloproteinase activity. J Cereb Blood Flow Metab, 2010. 30(2): p. 370–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bhattacharya A. and Biber K, The microglial ATP-gated ion channel P2X7 as a CNS drug target. Glia, 2016. 64(10): p. 1772–87. [DOI] [PubMed] [Google Scholar]
- 100.Skaper SD, Debetto P, and Giusti P, The P2X7 purinergic receptor: from physiology to neurological disorders. FASEB J, 2010. 24(2): p. 337–45. [DOI] [PubMed] [Google Scholar]
- 101.Ory D, Celen S, Gijsbers R, et al. , Preclinical Evaluation of a P2X7 Receptor-Selective Radiotracer: PET Studies in a Rat Model with Local Overexpression of the Human P2X7 Receptor and in Nonhuman Primates. J Nucl Med, 2016. 57(9): p. 1436–41. [DOI] [PubMed] [Google Scholar]
- 102.Janssen B, Vugts DJ, Wilkinson SM, et al. , Identification of the allosteric P2X7 receptor antagonist [(11)C]SMW139 as a PET tracer of microglial activation. Sci Rep, 2018. 8(1): p. 6580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Beaino W, Janssen B, Kooij G, et al. , Purinergic receptors P2Y12R and P2X7R: potential targets for PET imaging of microglia phenotypes in multiple sclerosis. J Neuroinflammation, 2017. 14(1): p. 259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kim E, Yang J, Beltran CD, et al. , Role of spleen-derived monocytes/macrophages in acute ischemic brain injury. J Cereb Blood Flow Metab, 2014. 34(8): p. 1411–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Prinz M. and Priller J, Tickets to the brain: role of CCR2 and CX3CR1 in myeloid cell entry in the CNS. J Neuroimmunol, 2010. 224(1–2): p. 80–4. [DOI] [PubMed] [Google Scholar]
- 106.Morganti JM, Jopson TD, Liu S, et al. , CCR2 antagonism alters brain macrophage polarization and ameliorates cognitive dysfunction induced by traumatic brain injury. J Neurosci, 2015. 35(2): p. 748–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gomez-Nicola D, Schetters ST, and Perry VH, Differential role of CCR2 in the dynamics of microglia and perivascular macrophages during prion disease. Glia, 2014. 62(7): p. 1041–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Peralta Ramos JM, Iribarren P, Bousset L, et al. , Peripheral Inflammation Regulates CNS Immune Surveillance Through the Recruitment of Inflammatory Monocytes Upon Systemic alpha-Synuclein Administration. Front Immunol, 2019. 10: p. 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Baufeld C, O’Loughlin E, Calcagno N, et al. , Differential contribution of microglia and monocytes in neurodegenerative diseases. J Neural Transm (Vienna), 2018. 125(5): p. 809–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Karlen SJ, Miller EB, Wang X, et al. , Monocyte infiltration rather than microglia proliferation dominates the early immune response to rapid photoreceptor degeneration. J Neuroinflammation, 2018. 15(1): p. 344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kim E. and Cho S, Microglia and Monocyte-Derived Macrophages in Stroke. Neurotherapeutics, 2016. 13(4): p. 702–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zarruk JG, Greenhalgh AD, and David S, Microglia and macrophages differ in their inflammatory profile after permanent brain ischemia. Exp Neurol, 2018. 301(Pt B): p. 120–132. [DOI] [PubMed] [Google Scholar]
- 113.Russo MV, Latour LL, and McGavern DB, Distinct myeloid cell subsets promote meningeal remodeling and vascular repair after mild traumatic brain injury. Nat Immunol, 2018. 19(5): p. 442–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Makinde HM, Cuda CM, Just TB, et al. , Nonclassical Monocytes Mediate Secondary Injury, Neurocognitive Outcome, and Neutrophil Infiltration after Traumatic Brain Injury. J Immunol, 2017. 199(10): p. 3583–3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Kim JY, Kim N, and Yenari MA, Mechanisms and potential therapeutic applications of microglial activation after brain injury. CNS Neurosci Ther, 2015. 21(4): p. 309–19.25475659 * We felt this review provided a succinct summary of a multitude of ways specific therapies can target microglia.
- 116.Kumar A, Alvarez-Croda DM, Stoica BA, et al. , Microglial/Macrophage Polarization Dynamics following Traumatic Brain Injury. J Neurotrauma, 2016. 33(19): p. 1732–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kumar A, Henry RJ, Stoica BA, et al. , Neutral Sphingomyelinase Inhibition Alleviates LPS-Induced Microglia Activation and Neuroinflammation after Experimental Traumatic Brain Injury. J Pharmacol Exp Ther, 2019. 368(3): p. 338–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Adams RA, Bauer J, Flick MJ, et al. , The fibrin-derived gamma377–395 peptide inhibits microglia activation and suppresses relapsing paralysis in central nervous system autoimmune disease. J Exp Med, 2007. 204(3): p. 571–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gao T, Chen Z, Chen H, et al. , Inhibition of HMGB1 mediates neuroprotection of traumatic brain injury by modulating the microglia/macrophage polarization. Biochem Biophys Res Commun, 2018. 497(1): p. 430–436. [DOI] [PubMed] [Google Scholar]
- 120.Ghosh M, Xu Y, and Pearse DD, Cyclic AMP is a key regulator of M1 to M2a phenotypic conversion of microglia in the presence of Th2 cytokines. J Neuroinflammation, 2016. 13: p. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gaire BP, Song MR, and Choi JW, Sphingosine 1-phosphate receptor subtype 3 (S1P3) contributes to brain injury after transient focal cerebral ischemia via modulating microglial activation and their M1 polarization. J Neuroinflammation, 2018. 15(1): p. 284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhou D, Ji L, and Chen Y, TSPO Modulates IL-4-Induced Microglia/Macrophage M2 Polarization via PPAR-gamma Pathway. J Mol Neurosci, 2020. 70(4): p. 542–549. [DOI] [PubMed] [Google Scholar]
- 123.Wen L, You W, Wang H, et al. , Polarization of Microglia to the M2 Phenotype in a Peroxisome Proliferator-Activated Receptor Gamma-Dependent Manner Attenuates Axonal Injury Induced by Traumatic Brain Injury in Mice. J Neurotrauma, 2018. 35(19): p. 2330–2340. [DOI] [PubMed] [Google Scholar]
- 124.Ji J, Xue TF, Guo XD, et al. , Antagonizing peroxisome proliferator-activated receptor gamma facilitates M1-to-M2 shift of microglia by enhancing autophagy via the LKB1-AMPK signaling pathway. Aging Cell, 2018. 17(4): p. e12774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhao R, Ying M, Gu S, et al. , Cysteinyl Leukotriene Receptor 2 is Involved in Inflammation and Neuronal Damage by Mediating Microglia M1/M2 Polarization through NF-kappaB Pathway. Neuroscience, 2019. 422: p. 99–118. [DOI] [PubMed] [Google Scholar]
- 126.Lan X, Han X, Li Q, et al. , Pinocembrin protects hemorrhagic brain primarily by inhibiting toll-like receptor 4 and reducing M1 phenotype microglia. Brain Behav Immun, 2017. 61: p. 326–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Miao H, Li R, Han C, et al. , Minocycline promotes posthemorrhagic neurogenesis via M2 microglia polarization via upregulation of the TrkB/BDNF pathway in rats. J Neurophysiol, 2018. 120(3): p. 1307–1317. [DOI] [PubMed] [Google Scholar]
- 128.Stowell RD, Sipe GO, Dawes RP, et al. , Noradrenergic signaling in the wakeful state inhibits microglial surveillance and synaptic plasticity in the mouse visual cortex. Nat Neurosci, 2019. 22(11): p. 1782–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Qiu Z, Lu P, Wang K, et al. , Dexmedetomidine Inhibits Neuroinflammation by Altering Microglial M1/M2 Polarization Through MAPK/ERK Pathway. Neurochem Res, 2020. 45(2): p. 345–353. [DOI] [PubMed] [Google Scholar]
- 130.Gao J, Sun Z, Xiao Z, et al. , Dexmedetomidine modulates neuroinflammation and improves outcome via alpha2-adrenergic receptor signaling after rat spinal cord injury. Br J Anaesth, 2019. 123(6): p. 827–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sharma M, Arbabzada N, and Flood PM, Mechanism underlying beta2-AR agonist-mediated phenotypic conversion of LPS-activated microglial cells. J Neuroimmunol, 2019. 332: p. 37–48. [DOI] [PubMed] [Google Scholar]
- 132.Jackson ML, Srivastava AK, and Cox CS Jr., Preclinical progenitor cell therapy in traumatic brain injury: a meta-analysis. J Surg Res, 2017. 214: p. 38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Walker PA, Shah SK, Jimenez F, et al. , Intravenous multipotent adult progenitor cell therapy for traumatic brain injury: preserving the blood brain barrier via an interaction with splenocytes. Exp Neurol, 2010. 225(2): p. 341–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Liu Y, Zhang R, Yan K, et al. , Mesenchymal stem cells inhibit lipopolysaccharide-induced inflammatory responses of BV2 microglial cells through TSG-6. J Neuroinflammation, 2014. 11: p. 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Galindo LT, Filippo TR, Semedo P, et al. , Mesenchymal stem cell therapy modulates the inflammatory response in experimental traumatic brain injury. Neurol Res Int, 2011. 2011: p. 564089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Walker PA, Bedi SS, Shah SK, et al. , Intravenous multipotent adult progenitor cell therapy after traumatic brain injury: modulation of the resident microglia population. J Neuroinflammation, 2012. 9: p. 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Bedi SS, Walker PA, Shah SK, et al. , Autologous bone marrow mononuclear cells therapy attenuates activated microglial/macrophage response and improves spatial learning after traumatic brain injury. J Trauma Acute Care Surg, 2013. 75(3): p. 410–6.23928737 ** We consider the references by Bedi in our cell therapy section to be of significant importance. These studies provide preclinical data on the potential benefit of cell therapy in the setting of TBI and the optimum timeframe to deliver the treatment for acute TBI.
- 138. Bedi SS, Hetz R, Thomas C, et al. , Intravenous multipotent adult progenitor cell therapy attenuates activated microglial/macrophage response and improves spatial learning after traumatic brain injury. Stem Cells Transl Med, 2013. 2(12): p. 953–60.24191266 ** We consider the references by Bedi in our cell therapy section to be of significant importance. These studies provide preclinical data on the potential benefit of cell therapy in the setting of TBI and the optimum timeframe to deliver the treatment for acute TBI.
- 139. Bedi SS, Aertker BM, Liao GP, et al. , Therapeutic time window of multipotent adult progenitor therapy after traumatic brain injury. J Neuroinflammation, 2018. 15(1): p. 84.29548333 ** We consider the references by Bedi in our cell therapy section to be of significant importance. These studies provide preclinical data on the potential benefit of cell therapy in the setting of TBI and the optimum timeframe to deliver the treatment for acute TBI.
- 140.Kota DJ, Prabhakara KS, van Brummen AJ, et al. , Propranolol and Mesenchymal Stromal Cells Combine to Treat Traumatic Brain Injury. Stem Cells Transl Med, 2016. 5(1): p. 33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Cox CS Jr., Baumgartner JE, Harting MT, et al. , Autologous bone marrow mononuclear cell therapy for severe traumatic brain injury in children. Neurosurgery, 2011. 68(3): p. 588–600.21192274 * We consider this clinical trial important, as it showed the safety of administering cell therapy in the pediatric population afflicted by acute TBI.