Figure 3. Many glycosyltransferases contribute to the generation of mucin-type O-glycan diversity.
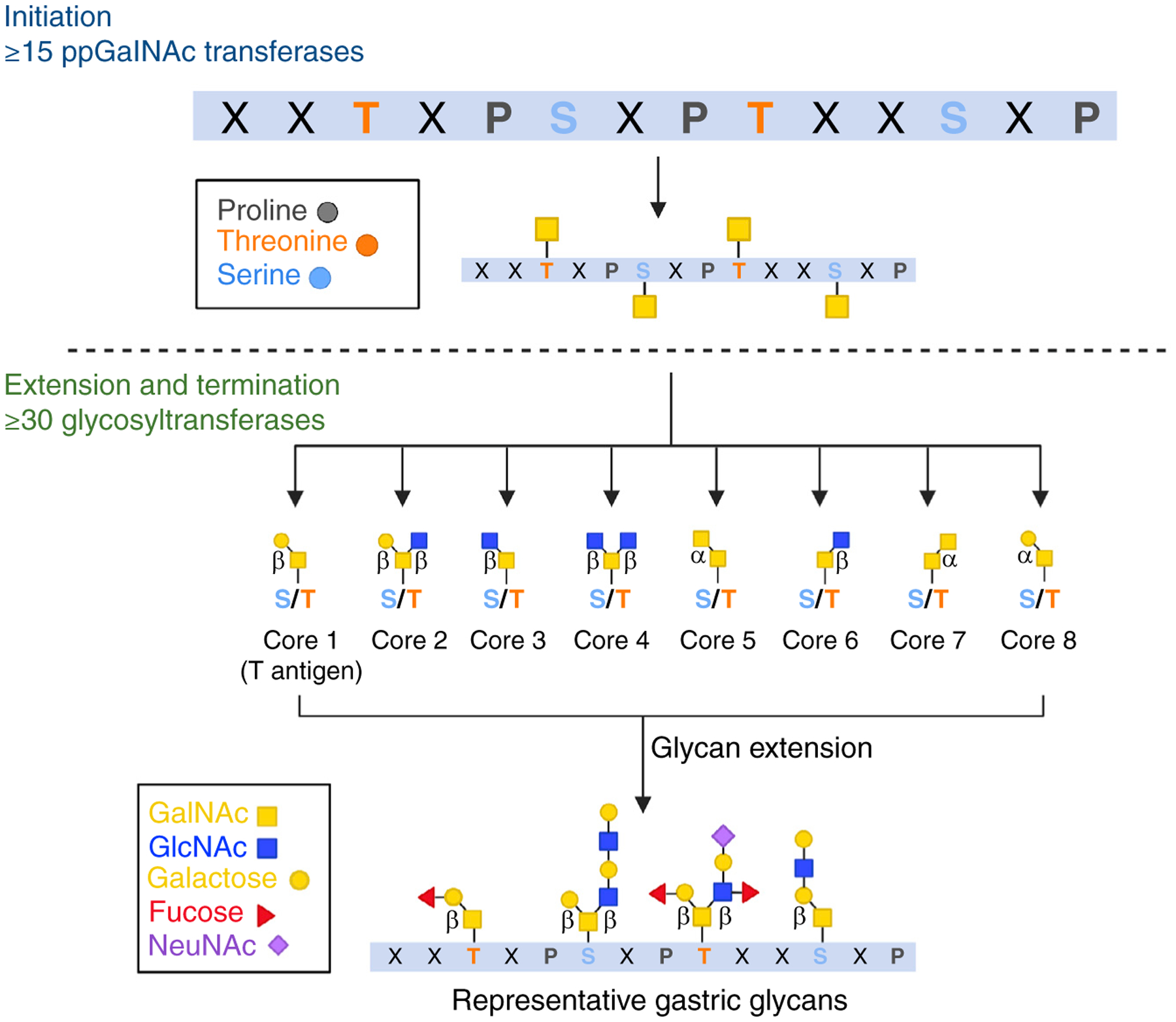
Serine and threonine residues act as potential sites of O-glycosylation within PTS domains. At least 15 ppGalNAc transferases can accomplish the first step, initiation, in which a GalNAc is transferred to a serine or threonine residue. As the O-glycosylated mucin moves through later compartments of the Golgi apparatus, glycan residues are further extended by at least 30 different additional glycosyltransferases with distinct donor and acceptor sugars using both α and β linkages. This combinatorial approach generates a plethora of diverse glycan structures based on eight core scaffolds, with cores 1–4 being the most common. Representative glycan structures present on human gastric mucin are shown.
