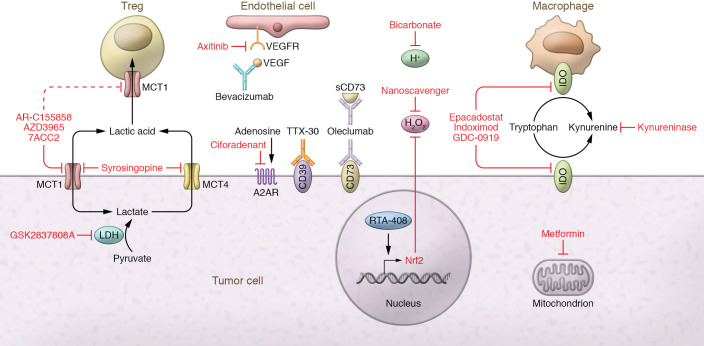
Figure 2. Metabolic alteration of the TME to improve cancer immunotherapy.
Alteration of the metabolic landscape of the tumor can be accomplished in many ways. Lactic acid production by tumor cells can be targeted using an inhibitor of LDH (GSK2837808A). Alternatively, lactic acid export by tumor cells could be targeted using MCT1 inhibitors (AZD3965 in clinical trials, 7ACC2 and AR-C155858 in preclinical work) or MCT4/MCT1 dual inhibitors (syrosingopine). MCT1 inhibitors may also block Treg import and usage of lactic acid, leading to diminished suppressive function and proliferation. Lactic acid lowers the pH of the TME, which can be counteracted through bicarbonate treatment. Tryptophan depletion and kynurenine production can be targeted by inhibition of IDO found on tumor cells and TAMs (epacadostat, indoximod, GDC-0919). Alternatively, kynurenine alone can be depleted using an enzyme engineered for its degradation (PEGylated kynureninase). Oxygen depletion can be targeted using VEGF inhibitors (bevacizumab) or VEGFR inhibitors (axitinib) to normalize tumor vasculature and improve tumor oxygenation. Metformin, a common diabetes drug, can be used to decrease tumor hypoxia, potentially through its action as a mitochondrial complex I inhibitor. ROS can be targeted through drugs promoting endogenous ROS scavengers (RTA-408 promoting Nrf2) or by addition of exogenous engineered ROS nanoscavengers. The production of adenosine can be targeted using monoclonal antibodies against CD39 (TTX-30) and CD73 (both membrane bound and soluble [sCD73]; oleclumab) or by small-molecule inhibition of the A2AR (ciforadenant).