Abstract
Although male Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) patients have higher Intensive Care Unit (ICU) admission rates and a worse disease course, a comprehensive analysis of female and male ICU survival and underlying factors such as comorbidities, risk factors, and/or anti-infection/inflammatory therapy administration is currently lacking. Therefore, we investigated the association between sex and ICU survival, adjusting for these and other variables. In this multicenter observational cohort study, all patients with SARS-CoV-2 pneumonia admitted to seven ICUs in one region across Belgium, The Netherlands, and Germany, and requiring vital organ support during the first pandemic wave were included. With a random intercept for a center, mixed-effects logistic regression was used to investigate the association between sex and ICU survival. Models were adjusted for age, Acute Physiology and Chronic Health Evaluation II (APACHE II) score, comorbidities, and anti-infection/inflammatory therapy. Interaction terms were added to investigate effect modifications by sex with country and sex with obesity. A total of 551 patients (29% were females) were included. Mean age was 65.4 ± 11.2 years. Females were more often obese and smoked less frequently than males (p-value 0.001 and 0.042, respectively). APACHE II scores of females and males were comparable. Overall, ICU mortality was 12% lower in females than males (27% vs 39% respectively, p-value < 0.01) with an odds ratio (OR) of 0.62 (95%CI 0.39–0.96, p-value 0.032) after adjustment for age and APACHE II score, 0.63 (95%CI 0.40–0.99, p-value 0.044) after additional adjustment for comorbidities, and 0.63 (95%CI 0.39–0.99, p-value 0.047) after adjustment for anti-infection/inflammatory therapy. No effect modifications by sex with country and sex with obesity were found (p-values for interaction > 0.23 and 0.84, respectively). ICU survival in female SARS-CoV-2 patients was higher than in male patients, independent of age, disease severity, smoking, obesity, comorbidities, anti-infection/inflammatory therapy, and country. Sex-specific biological mechanisms may play a role, emphasizing the need to address diversity, such as more sex-specific prediction, prognostic, and therapeutic approach strategies.
Subject terms: Diseases, Medical research, Pathogenesis, Risk factors
Introduction
In early 2020, a novel β-coronavirus causing Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) rapidly spread worldwide, resulting in a pandemic with global impact with an important proportion of the hospitalized patients requiring supportive treatment in the Intensive Care Unit (ICU)1,2. By now, risk factors such as age, smoking, cardiovascular and pulmonary comorbidities have been ascertained to influence fatality rates in hospitalized patients strongly3. Although Coronavirus Disease 2019 (COVID-19) infects both sexes at similar incidence, case-fatality rates are lower for females (7%) than males (10%), resulting in a marked male-to-female case fatality ratio ranging from 1.6 to 2.8, according to the Global Health 50/50 data tracker4,5. Furthermore, a systematic review and meta-analysis of more than 3 million cases demonstrated that male sex was associated with higher odds ratios of requiring ICU admission and overall mortality6. On the other hand, an Italian multicenter study revealed higher ICU admission rates for males than females7,8. Furthermore, multi-organ failure in the ICU has appeared to develop more favorable in surviving females than males9. Previous research confirmed the findings that male patients predominate in the ICU and receive more supportive treatment than females10–12.
Sex contributed to disparities in vulnerability, incidence, and case-fatality rates of various diseases in the past4,13.
It is assumed that sex differences affect viral susceptibility, response to the virus, disease course, and (side-) effects of initiated therapy, emphasizing why sex aspects and sex-specific data analyses should be implemented in clinical studies14,15. We recently demonstrated that current clinical trials on pharmacological therapies for COVID-19 rarely report sex-stratified analyses16, which illustrates that sex differences are infrequently taken into account.
Although some studies have depicted higher mortality rates in male patients6,17–19, these studies have been performed in heterogeneous populations with clinical diversity, such as a broad range of disease courses, ranging from mild symptoms to respiratory insufficiency requiring ICU admission20. More importantly, other baseline risk factors known to be associated with poor outcome, such as age, comorbidities, disease severity, and therapy, are often not taken into account21. To conclude, a comprehensive analysis of female and male survival of COVID-19 in the ICU and underlying factors currently lacks in the literature22–24, where sex differences in survival and the role of potentially confounding factors remain unknown7,8. Therefore, we investigated the association between sex and ICU survival in a Western European cohort of SARS-CoV-2 infected patients, adjusting for age, disease severity, obesity, smoking, comorbidities, and anti-infection/inflammatory therapy.
Results
From the 2nd of March 2020 to the 12th of August 2020, 551 patients with COVID-19 pneumonia were admitted to seven ICUs mentioned above in Western Europe (Fig. 1), 434 (79%) were mechanically ventilated. During illness, 18 (3%) patients were transferred within the Euregio and thus admitted to two or three of the Euregio ICUs (due to lack of bed availability and/or tertiary care referral for extracorporeal membrane oxygenation consideration). The number of females in the whole cohort was 159 (29%). The mean age was comparable between females and males (64.1 ± 12.6 vs 66.0 ± 10.5 years, p-value 0.095), as were the presence of dyslipidemia, diabetes mellitus, hypertension, chronic liver disease, chronic lung disease, and chronic renal disease (Table 1, p-values > 0.05). However, females were more often obese than males (42% vs 28%, p-value 0.001) and reported smoking less often than males (15% vs 22%, p-value 0.042). Acute Physiology And Chronic Health Evaluation II (APACHE II) scores did not differ between females and males (15.7 ± 5.2 vs 16.3 ± 5.6, p-value 0.305).
Figure 1.
Flow chart.
Table 1.
ICU admission characteristics stratified for females and males of the full Euregio Intensive Care cohort.
| Females | Males | p-value | |
|---|---|---|---|
| Number of patients | 159 | 392 | |
| Age, years | 64.1 ± 12.6 | 66.0 ± 10.5 | 0.095 |
| Height, m | 1.63 ± 0.08 | 1.78 ± 0.08 | < 0.001 |
| Weight, kg | 80.5 ± 17.7 | 90.0 ± 16.1 | < 0.001 |
| Body mass index, kg/m2 | 30.1 ± 6.4 | 28.6 ± 4.7 | 0.007 |
| Obesity, n (%) | 66 (42) | 109 (28) | 0.001 |
| Dyslipidemia, n (%) | 41 (26) | 108 (28) | 0.500 |
| Diabetes Mellitus, n (%) | 40 (25) | 101 (26) | 0.882 |
| Hypertension, n (%) | 76 (48) | 184 (47) | 0.805 |
| Smoking, n (%) | 24 (15) | 88 (22) | 0.042 |
| Chronic liver disease, n (%) | 1 (1) | 3 (1) | 1.000a |
| Chronic lung disease, n (%) | 34 (21) | 67 (17) | 0.238 |
| Chronic renal disease, n (%) | 23 (15) | 45 (12) | 0.334 |
| Patients admitted from the emergency department/hospital ward/by transport, n | 54/77/28 | 130/200/62 | 0.816 |
| Patients from Belgium/ the Netherlands/Germany, n | 60/77/22 | 118/233/41 | 0.061 |
| APACHE II score | 15.7 ± 5.2 | 16.3 ± 5.6 | 0.305 |
| Antibacterial therapy, n (%) | 145 (91) | 378 (96) | 0.011 |
| Antiviral medication, n (%) | 0.527a | ||
| Oseltamivir, n (%) | 6 (4) | 8 (2) | |
| Lopinavir/ritonavir, n (%) | 5 (3) | 13 (3) | |
| (Hydroxy)chloroquine, n (%) | 82 (52) | 234 (60) | 0.081 |
| Remdesivir, n (%) | 2 (1) | 0 (0) | 0.083a |
| Interleukin inhibitors, n (%) | 6 (4) | 15 (4) | 0.972 |
| Steroids, n (%) | 56 (35) | 116 (30) | 0.223 |
Data are presented as mean ± SD, median [IQR], or percentages. P-values for differences between sex are tested by independent Student's T-Test, Mann–Whitney U test or Chi-Square, as appropriate unless otherwise specified: aFisher's exact test. ICU, Intensive Care Unit. The comprehensive data for the full cohort were complete, except missings for height (n = 27), weight (n = 33), BMI (n = 37), obesity (n = 20), dyslipidemia (n = 108), hypertension (n = 1), smoking (n = 96), antiviral medication (n = 3), interleukin inhibitors (n = 1), and steroids (n = 4).
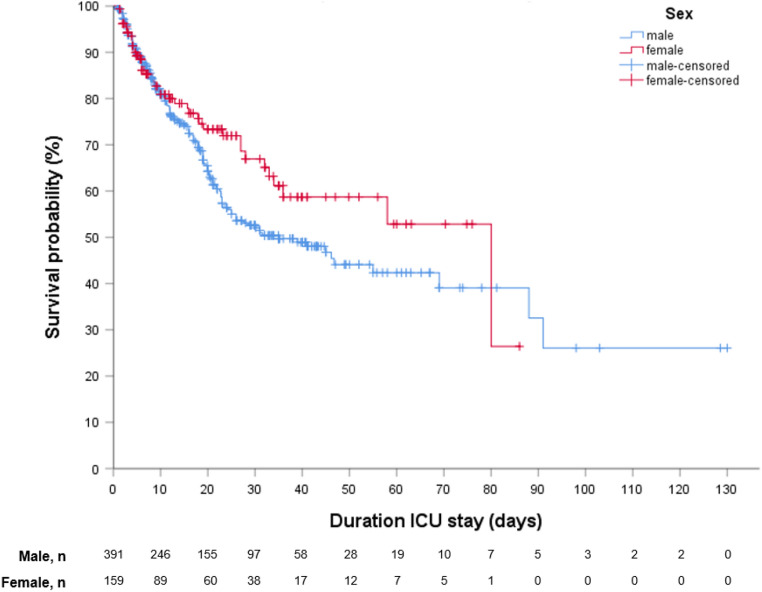
ICU length of stay was comparable between females and males (12.4 [5.0–28.0] vs 16.0 [7.0–30.0] days, p-value 0.347). During ICU stay, females required less often invasive mechanical ventilation (72% vs 82% respectively, p-value 0.010) and antibacterial therapy than males (91% vs 96%, respectively, p-value 0.011). Administration of other anti-infection/inflammatory drugs did not differ between the sexes (Table 1). The ICU mortality rate was 12% lower in females than males (27% vs 39%, p-value < 0.008) (Table 2, Fig. 2). The Kaplan–Meier survival curves showed that more females survived in the ICU than males, while the curves crossed around 80 days of admission (although with a very low number of events) (Fig. 2).
Table 2.
ICU outcomes of the Euregio Intensive Care cohort stratified for females and males.
| Females | Males | p-value | |
|---|---|---|---|
| Number of patients | 159 | 392 | |
| ICU death, n (%) | 43 (27) | 153 (39) | 0.008 |
| Length of ICU stay, days | 12.4 [5.0–28.0] | 16.0 [7.0–30.0] | 0.347 |
| Invasive mechanical ventilation, n (%) | 114 (72) | 320 (82) | 0.010 |
| Length of invasive mechanical ventilation, days | 15.8 [8.20–27.0] | 16.5 [8.7–27.9] | 0.477 |
Data are presented as median [IQR], or percentages. P-values for differences between sex are tested by the Mann–Whitney U test or Chi-Square, as appropriate. ICU, Intensive Care Unit. The comprehensive data for the full cohort were complete, except missings for length of ICU stay (n = 1) and length of invasive mechanical ventilation (n = 4).
Figure 2.
Kaplan–Meier survival estimate by sex ICU, Intensive Care Unit. The Kaplan–Meier survival curves show that more females survive the ICU than males, while the curves cross around 80 days with a very low number of events by then. Number at risk (n) = 550 as 1 patient missed data on duration of ICU stay.
In a mixed-effects crude logistic regression model with a random center effect, females had a lower odds ratio for mortality than males (OR 0.59 (95%CI 0.39–0.89), Table 3, model 1), suggesting higher ICU survival rates in females than males. Adjustment for age and APACHE II score (OR 0.62 (95%CI 0.39–0.96), model 2), and additional adjustment for the presence of obesity, dyslipidemia, diabetes mellitus, hypertension, smoking, chronic liver disease, chronic lung disease, and chronic renal disease (OR 0.63 (95%CI 0.40–0.99), model 3), or antibacterial therapy, antiviral medication, (hydroxy)chloroquine, remdesivir, interleukin inhibitors, and steroids (OR 0.63 (95%CI 0.39–0.99), model 4) did not change the results. Similar results were observed in the subgroup of mechanically ventilated patients (n = 434) (Table 3). Between-center variances in model intercepts were not statistically significantly different (p-values for models 2, 3 and 4 ≥ 0.17), which means that baseline characteristics of sexes did not explain the observed sex differences in mortality. When adding interaction terms for sex and country and sex and obesity to the models, no effect modifications were observed (p-values for interaction > 0.23 and 0.84, respectively). A sensitivity analysis with missing data handled by multiple imputation showed similar results.
Table 3.
The association between sex and ICU death by mixed-logistic regression analyses.
| Full cohort n = 551 | Mechanically ventilated subcohort n = 434 | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Model 1. The crude model with a random intercept for hospital | 0.59 | 0.39–0.89 | 0.012 | 0.56 | 0.36–0.89 | 0.014 |
| Model 2. Model 1 + age and APACHE II score | 0.62 | 0.39–0.96 | 0.032 | 0.57 | 0.35–0.92 | 0.023 |
| Model 3. Model 2 + obesity, dyslipidemia, diabetes mellitus, hypertension, smoking, chronic liver disease, chronic lung disease and chronic renal disease | 0.63 | 0.40–0.99 | 0.044 | 0.61 | 0.37–1.01 | 0.052 |
| Model 4. Model 2 + antibacterial therapy, antiviral medication, (hydroxy)chloroquine, remdesivir, interleukin inhibitors, and steroids | 0.63 | 0.39–0.99 | 0.047 | 0.58 | 0.35–0.96 | 0.036 |
Data are odds ratios (OR) with 95% confidence intervals (95% CI) for females compared to males (as reference). A lower OR indicates an increased survival rate for females. Missings (for the full cohort: obesity (n = 20), dyslipidemia (n = 108), hypertension (n = 1), smoking (n = 96), antiviral medication (n = 3), interleukin inhibitors (n = 1), and steroids (n = 4)) were included in model 3 and 4 as separate category.
In addition, sensitivity analyses of excluding transported patients displayed similar effect estimates, although statistical power was somewhat reduced (Supplementary Table S1).
Discussion
Although patients are individuals, traditionally, we tend to categorize them according to their disease or condition and treat them in the same "diagnosis category" using one-size-fits-all interventions. However, how a disease may unfold in an individual patient has many dimensions, and heterogeneity has major implications on disease course and outcome25. For COVID-19, heterogeneity in the course of the disease and complications has been observed9,26, ranging from typical flu-like symptoms to critical illness requiring intensive care admission and death27,28.
In this cohort study of 551 COVID-19 patients admitted to the ICUs of seven hospitals in three Western European countries, we demonstrated that females had a 40% greater chance to survive ICU stay than males, independent of age, the severity of acute critical illness, obesity, smoking, major comorbidities, administered anti-infection/inflammatory therapy, and country. The results were similar for the subgroup of invasively mechanically ventilated patients. No effect modifications for sex with country and sex with obesity were present. Secondly, we observed that females were the minority in the Euregio cohort, representing 29% of the study population.
The prevalence of females in our study is in line with the reported prevalence in previous COVID-19 studies in ICU patients7. In a multicenter observational study in Italian ICU patients8, 20.1% (95%CI 18.9–21.3) of the study population was female with a median age of 64 years, which is in line with the age in our study. However, the reported mortality of approximately 50% is higher than in our study, which may relate to the higher prevalence of comorbidities in their cohort. Nevertheless, in agreement with our findings, they found that the male sex was significantly associated with mortality (HR 1.57 95%CI 1.31–1.88), although they did not adjust for the severity of disease.
Overall, evidence points towards a slightly lower prevalence of symptomatic COVID-19 in females than males (45% vs 55%, respectively)29, with males having a consistently three times higher odds for ICU admission and once admitted an up to 40% higher mortality rate compared with females, both in line with our findings6. Notably, these findings are not unique for COVID-19. In fact, higher mortality rates for males were also observed in previous Middle East Respiratory Syndrome Coronavirus and Severe Acute Respiratory Syndrome Coronavirus 1 outbreaks30,31. Several studies have demonstrated that elderly patients with comorbidities are at increased risk of dying from COVID-1932–34. Additionally, underlying health conditions such as cardiovascular disease, hypertension, diabetes mellitus, smoking, and obesity were associated with an increased risk of mortality35–37. Our study shows a higher odds of mortality for males, as other studies do, but is distinctive since results are independent of age, smoking, obesity, comorbidities, APACHE II scores (i.e., the classification system used to assess disease severity)38, anti-infection/inflammatory therapy, and country, indicating that sex might be associated with ICU outcome independently of disease severity at ICU admission.
Although COVID-19 seems to be linked to multiple "traditional" risk factors, its presentation is multidimensional, with sex and gender being essential determining factors. Although it had been suggested that the sex disparities in COVID-19 were due to higher smoking and comorbidity rates observed in males39, we show that the association between sex and ICU outcome is independent of disease severity, “traditional risk factors,” and treatments. As treatment could be considered as a mediator, instead of a confounder, in the reported models, the results suggest that treatments evaluated are not a clinically and statistically significant contributor to the causal pathway between sex and ICU outcome. Even more striking is the higher prevalence of obesity seen in females in our cohort, which has been associated with worse outcomes in earlier studies, while it did not affect the better survival of females compared to males in our study.
These findings support the theory that the pathophysiology of SARS-COV-2 infection may differ between both sexes in the ICU setting9,16. Indeed, evidence on several sex-specific interacting mechanisms on immunological, hormonal, and cardiovascular pathophysiology is accumulating4,14,29,40–50. Since many genes involved in the immunological response to infection are present on the X chromosome, the XX and XY genetic constitutions could also potentially contribute to COVID-19 severity40,48. Females show a more rapid and aggressive immune response to pathogens with a lower degree of systemic inflammation, which facilitates viral clearance41,51. Moreover, females have a more robust T-cell activation during SARS-CoV-2 infection, whereas males have higher plasma levels of innate immune cytokines and increased non-classical monocyte cell populations induction. Both observations are correlated with increased severity in males49,50. In addition, female and male steroid and sex hormones could play a contributory role in pathogenesis. Estrogen in females, for instance, can have immune-enhancing effects, while testosterone in males can exert immune-suppressive effects. Furthermore, the expression of receptors that determine viral cell entry, such as transmembrane protease serine 2 (TMPRSS2) and angiotensin-converting enzyme (ACE), is affected by sex hormones and lower in females than in males40–44. TMPRSS2 is enhanced by testosterone and may play a role in delayed viral clearance41,42, whereas the ACE2 receptor, exerting protective effects in the heart, lungs, kidneys, and guts by deactivating the effects of the renin-angiotensin system (RAS)51, is encoded on the X chromosome and downregulated by estrogens. Thus, females show a reduced propensity to upregulate RAS activity in COVID-19, which could contribute to higher disease severity in males, as RAS overactivation contributes to pathogenesis in cardiovascular disease and potentially COVID-1951.
Our study shows for the first time that, independent of cardiovascular comorbidities, females have a survival benefit for COVID-19 once admitted to the ICU. Sex differences in the prevalence of subclinical and yet undiagnosed underlying cardiovascular comorbidities may still play a role, as we cannot entirely exclude residual confounding21, while our adjustments were comprehensive. However, it is unlikely that residual confounding thoroughly explains the observed sex difference.
Strengths and limitations
Several studies have shown that females have higher survival rates in heterogenic population-based data sets4. Our study, however, is the first to demonstrate that a higher survival rate of females is maintained after admission to a Western European ICU, and more importantly, independent of age, disease severity, obesity, smoking, comorbidities, anti-infection/inflammatory therapy, and country. Sensitivity analyses, excluding transported patients to and out of Euregio, showed similar results, which reduces bias due to loss of ICU follow-up beyond Euregio. In addition, the identification of transports within Euregio reduces bias due to including the same patient twice in the models. Although we collected variables from healthcare systems, our data collection was complete (models 1 and 2), except for a few missings for some potential confounders (obesity and hypertension), while smoking and dyslipidemia only were less complete (> 5% missings) (models 3). Therefore, we additionally used multiple imputation to handle missing data appropriately52–55. The data collection process met high standards, and collected healthcare data were of high quality using a predefined protocol addressing the present hypothesis56. Moreover, we used multivariable adjustments to address a comprehensive set of potential confounders. Nevertheless, the study cannot rule out that residual confounding has biased the reported associations21,57,58. We did not follow-up on patients over a predefined time period and classified survivors as those who were discharged from the ICU or transported.
As patients may have died after ICU discharge or transport, we feel that a survival analysis including time cannot be appropriately performed and would present invalid results, which is a limitation of the study. Consequently, no conclusion can be drawn on COVID-19 progression in the ICU based on our data. We recognize that our findings are limited to the ICU population of the Euregio, thereby limiting generalization to other contexts outside the ICU, such as other patient populations (e.g., from national registries or hospitalized) and regions59,60. Admission to the ICU is the result of a selection process, which depends on many factors, including those potentially associated with sex and possibly causes index-event bias61,62. If so, the observed association between sex and ICU survival could function in this selection process. Thus, as an alternative to the pathophysiological explanation for the observed sex differences in ICU outcome as discussed above, the results could also be explained by doctors’ decisions regarding admission of patients to the ICU. Unfortunately, our dataset did not include information on the source population (i.e., all patients admitted for COVID-19 to the seven hospitals), and we thus cannot investigate whether selection has occurred. In addition, the results cannot be generalized beyond the ICU. As the large majority of inhabitants of Euregio are of Caucasian descent, diversity due to ethnicity within our cohort was too low for subanalysis. This variable was not considered for the collection, while it was not registered in a standardized way within each of the hospitals. Finally, the observational study design limits to conclude with regard to causality and the relation with subclinical yet undiagnosed underlying cardiovascular disease.
In this study in the Euregio, we demonstrate that females, once admitted to the ICU with COVID-19 pneumonia, have a 40% higher survival rate relative to males and that this association is independent of age, disease severity, obesity, smoking, comorbidities, anti-infection/inflammatory therapy, and country of residence. Understanding the relation between sex and COVID-19 implies recognizing diversity in the role of both biological and social factors in the risk of infection and disease, clinical presentation, the severity of outcomes, and patient selection at the individual as well as population levels. A sex-specific prediction, prognostic and therapeutic approach and sex-stratified analyses strategies should be implemented in future ICU studies, while further studies on sex-specific mechanistic pathways in SARS-CoV-2 are warranted.
Methods
The Euregio Intensive Care COVID cohort, part of the Euregio Covid Data Platform (CoDaP) project, was initiated in early March, at the beginning of the first wave of the COVID-19 pandemic. Seven neighboring ICUs (Supplementary Table S2) in Belgium, the Netherlands, and Germany collaborated and planned to share their data in a predesigned way63.
We consecutively included patients with COVID-19 pneumonia and respiratory failure admitted to the ICU of any of the seven hospitals between the 2nd of March 2020 and the 12th of August 2020 (Fig. 1). At the time of admission, all patients presented with signs and symptoms of viral pneumonia. The diagnosis was confirmed by a positive PCR for SARS-CoV-2 and/or (for the Netherlands only) a positive score on chest CT scan of 4–5 based on the COVID-19 Reporting and Data System (CO-RADS) score as confirmed by a radiologist64. Patient admission occurred via the emergency department, non-ICU wards, or from other (international) ICUs, in the latter case either for tertiary care referral or due to lack of bed availability. Follow-up ended when patients were either discharged from ICU or died, from the 11th of March to the 2nd of September.
Data collection
At the beginning of the pandemic, the number of variables was determined in the focus of interest. These included baseline demographic and clinical characteristics, laboratory values, interventions, and outcome variables, which were predefined and routinely obtained during patients' stay in the above-mentioned ICUs. The main outcome variable for the present study was ICU death. Variables were collected mostly retrospectively and pseudo-anonymized at the collecting hospital. Pseudo-anonymization of data is a widely accepted method that aims to secure the patient's privacy about his/her healthcare data while ensuring the possibility to re-collect data at the dispatching hospital. Subsequently, data were shared with Maastricht UMC+, the coordinating center within CoDaP, using electronically secured data transfer methods and stored on a secured hospital hard drive. Data access was only permitted for the primary investigators.
Data cleaning started by checking whether each variable of the study protocol was present in each of the seven datasets. Next, each hospital dataset was standardized (i.e., standardization of the variable names, characteristics, and units). When a variable was missing or inconsistencies were encountered, contact with investigators of the dispatching hospital led to re-collecting, re-calculation, and re-sending of those variables65. The seven datasets were then merged into one dataset. Patients transferred between the ICUs of the seven Euregio hospitals were identified. Their whole ICU stay was attributed to the primary admitting ICU to prevent duplicate patient data and missing data by combining baseline and outcome data. Finally, each of the pre-final Euregio cohort variables dataset was evaluated for outliers through running queries, which was checked with the source dataset of the dispatching hospital and corrected if possible and appropriate or defined as missing. Obesity was defined as a body mass index (BMI) equal to or larger than 30 kg/m2. Smoking was defined as either active smoking or a history of smoking. Comorbidities were defined either as a history of a medical diagnosis or the actual use of medication for such medical diagnosis before ICU admission.
Statistical analyses
IBM SPSS Statistics version 25 (IBM corporation, NY, USA) was used for all analyses. The full cohort was categorized into females and males, and data were presented. The number of missings per variable is reported in the table legends. Descriptive statistics were performed on available data only. Differences between females and males were analyzed using the independent Student's T-test, Mann–Whitney U test, Chi-Square, or Fisher's exact test, as appropriate. With a random intercept for a center, mixed-effects logistic regression was used to investigate the association between sex and ICU survival. Crude models were first adjusted for age and APACHE II score, then for obesity, dyslipidemia, diabetes mellitus, hypertension, smoking, chronic liver disease, chronic lung disease, and chronic renal disease21, and eventually for antibacterial therapy, antiviral medication such as oseltamivir, ritonavir/lopinavir, (hydroxy)chloroquine and remdesivir, interleukin inhibitors, and steroids. Confounders were treated as continuous or categorical variables, and missing data were included as a separate category. As this method could lead to bias in observational studies52–55, a sensitivity analysis was performed by handling missing data by multiple imputation. Missing values in the variables included in the model were explored, and the imputation number was based on the percentage of patients with at least one missing value. Predictive mean matching was the method of choice, and variables with missing data, auxiliary variables, and outcome variables were added to the model. Effect modifications by sex with country and sex with obesity were investigated by adding interaction terms for categories of sex and country and sex and obesity to the models. Analyses were performed for the full Euregio cohort and the mechanically ventilated patients of the cohort. Subsequently, sensitivity analyses were performed by excluding patients who were transported from a hospital outside the Euregio to our participating Euregio hospitals (i.e., due to missing baseline data), and by additionally excluding patients who were transported out of the Euregio (i.e., due to missing ICU follow-up data from hospitals beyond Euregio). Finally, for illustration, Kaplan–Meier survival curves were created. A two-sided p-value of < 0.05 and a p-interaction of < 0.1 were considered statistically significant.
Ethical approval
Ethical approval was obtained from the medical ethics committee (METC 2020–1565/3 00 523) of Maastricht UMC+. The study was performed in accordance with the General Data Protection Regulation (GDPR) act and the national data privacy laws. Patient data were collected according to good clinical practice and in accordance with relevant guidelines and regulations. Informed consent was waived by the METC of Maastricht UMC+. However, each of the participating hospitals had its own policy and approach. For example, in Maastricht UMC+, the board of directors adopted a policy to inform patients and ask their consent to use collected data63. Data sharing agreements between Maastricht UMC+ and each hospital were drawn up by legal officers of Maastricht UMC+ and Clinical Trial Center Maastricht (CTCM) and subsequently tailored to each hospital. Investigators, heads of ICU departments, and the hospital board of directors of the Maastricht UMC+ and the other hospitals signed the final data-sharing agreement.
Supplementary Information
Author contributions
Conceptualization and study design: D.M., I.C.C.v.d.H., B.C.T.v.B., G.M., C.G.-D.; Data curation: B.C.T.v.B., D.A.M.M., CoDAP investigators; Formal Analysis: B.C.T.v.B., D.A.M.M.; Funding acquisition: D.M., G.M., I.C.C.v.d.H., B.S., B.C.T.v.B.; Investigation: A.H., J.M.-S., C.I.E.S.; Methodology: B.C.T.v.B., I.C.C.v.d.H., S.A.E.P., C.G.-D.; Resources: CoDaP investigators; Supervision: B.C.T.v.B., I.C.C.v.d.H., C.G.-D.; Validation: B.C.T.v.B., D.A.M.M.; Visualisation: B.C.T.v.B., D.A.M.M.; Writing—original draft: C.G.-D., D.A.M.M., B.C.T.v.B.; Writing—review & editing: D.A.M.M., B.C.T.v.B., B.S., J.M.-S., A.H., C.I.E.S., S.A.E.P., W.N.K.A.v.M., I.C.C.v.d.H., G.M., D.M., C.G.-D., CoDaP Investigators.
Funding
Our study was supported by the "Interreg Euregio Meuse-Rhine" (Covid Data Platform (CoDaP) Grant: Interreg-EMR 187). Funding sources were not involved in the study design, data collection, data analysis, data interpretation, writing process, and decision to submit for publication. Researchers were independent of funders, and all authors had full data access.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available due to data sharing agreements of the participating hospitals. Individual patient data and the pseudo-anonymized dataset will not be made available to others. Only data for the full cohort or a particular subcohort will be published and shared after the provision of a research proposal and signed data access agreement of each participating hospital.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A list of authors and their affiliations appears at the end of the paper.
Contributor Information
Daniek A. M. Meijs, Email: daniek.meijs@mumc.nl
CoDaP investigators:
Nanon F. L. Heijnen, Johannes Bickenbach, Meta C. E. van der Woude, Anne Raafs, Sander M. J. van Kuijk, Luc J. M. Smits, Emma B. N. J. Janssen, Noёlla Pierlet, Ben Goethuys, Jonas Bruggen, Gilles Vermeiren, Hendrik Vervloessem, Mark M. G. Mulder, Marcel Koelmann, Julia L. M. Bels, Laura Bormans-Russell, Micheline C. D. M. Florack, Willem Boer, and Margot Vander Laenen
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-04531-x.
References
- 1.World Health Organization. Coronavirus Disease 2019 (COVID-19): Situation Report 141. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200609-covid-19-sitrep-141.pdf?sfvrsn=72fa1b16_2 (2020).
- 2.World Health Organization.WHO Director General's Opening Remarks at the Media Briefing on COVID-19. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---5-june-2020 (2020).
- 3.Leung C. Clinical features of deaths in the novel coronavirus epidemic in China. Rev. Med. Virol. 2020;30:e2103. doi: 10.1002/rmv.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gebhard C, Regitz-Zagrosek V, Neuhauser HK, Morgan R, Klein SL. Impact of sex and gender on COVID-19 outcomes in Europe. Biol. Sex Differ. 2020;11:29. doi: 10.1186/s13293-020-00304-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Launer J. Burnout in the age of COVID-19. Postgrad. Med. J. 2020;96:367–368. doi: 10.1136/postgradmedj-2020-137980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peckham H, et al. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat. Commun. 2020;11:6317. doi: 10.1038/s41467-020-19741-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grasselli G, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grasselli G, et al. Risk factors associated with mortality among patients With COVID-19 in Intensive Care Units in Lombardy, Italy. JAMA Intern. Med. 2020;180:1345–1355. doi: 10.1001/jamainternmed.2020.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bels JLM, et al. Decreased serial scores of severe organ failure assessments are associated with survival in mechanically ventilated patients; the prospective Maastricht Intensive Care COVID cohort. J. Crit. Care. 2020;62:38–45. doi: 10.1016/j.jcrc.2020.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samuelsson C, Sjoberg F, Karlstrom G, Nolin T, Walther SM. Gender differences in outcome and use of resources do exist in Swedish intensive care, but to no advantage for women of premenopausal age. Crit. Care. 2015;19:129. doi: 10.1186/s13054-015-0873-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valentin A, Jordan B, Lang T, Hiesmayr M, Metnitz PG. Gender-related differences in intensive care: A multiple-center cohort study of therapeutic interventions and outcome in critically ill patients. Crit. Care Med. 2003;31:1901–1907. doi: 10.1097/01.CCM.0000069347.78151.50. [DOI] [PubMed] [Google Scholar]
- 12.Fowler RA, et al. Sex-and age-based differences in the delivery and outcomes of critical care. CMAJ. 2007;177:1513–1519. doi: 10.1503/cmaj.071112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehta S, et al. Gender parity in critical care medicine. Am. J. Respir. Crit. Care Med. 2017;196:425–429. doi: 10.1164/rccm.201701-0076CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bischof E, Wolfe J, Klein SL. Clinical trials for COVID-19 should include sex as a variable. J. Clin. Invest. 2020;130:3350–3352. doi: 10.1172/JCI139306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bischof E, et al. Towards precision medicine: Inclusion of sex and gender aspects in COVID-19 clinical studies-acting now before it is too late-a joint call for action. Int. J. Environ. Res. Public Health. 2020;17:3715. doi: 10.3390/ijerph17103715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schiffer VMMM, et al. The "sex gap" in COVID-19 trials: A scoping review. EClinicalMedicine. 2020;29:100652. doi: 10.1016/j.eclinm.2020.100652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Penna C, Mercurio V, Tocchetti CG, Pagliaro P. Sex-related differences in COVID-19 lethality. Br. J. Pharmacol. 2020;177:4375–4385. doi: 10.1111/bph.15207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahrenfeldt LJ, Otavova M, Christensen K, Lindahl-Jacobsen R. Sex and age differences in COVID-19 mortality in Europe. Res Sq. 2020 doi: 10.21203/rs.3.rs-61444/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohamed MO, et al. Sex differences in mortality rates and underlying conditions for COVID-19 deaths in England and Wales. Mayo Clin Proc. 2020;95:2110–2124. doi: 10.1016/j.mayocp.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barbateskovic M, et al. A new tool to assess Clinical Diversity In Meta-analyses (CDIM) of interventions. J. Clin. Epidemiol. 2021;135:29–41. doi: 10.1016/j.jclinepi.2021.01.023. [DOI] [PubMed] [Google Scholar]
- 21.Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. 3. Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 22.Conti P, Younes A. Coronavirus COV-19/SARS-CoV-2 affects women less than men: Clinical response to viral infection. J. Biol. Regul. Homeost. Agents. 2020;34:339–343. doi: 10.23812/Editorial-Conti-3. [DOI] [PubMed] [Google Scholar]
- 23.Cai H. Sex difference and smoking predisposition in patients with COVID-19. Lancet Respir. Med. 2020;8:e20. doi: 10.1016/S2213-2600(20)30117-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wenham C, Smith J, Morgan R. COVID-19: The gendered impacts of the outbreak. Lancet. 2020;395:846–848. doi: 10.1016/S0140-6736(20)30526-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vincent JL, et al. COVID-19: What we've done well and what we could or should have done better-the 4 Ps. Crit. Care. 2021;25:40. doi: 10.1186/s13054-021-03467-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Gassel RJJ, et al. High prevalence of pulmonary sequelae at 3 months after hospital discharge in mechanically ventilated COVID-19 survivors. Am. J. Respir. Crit. Care Med. 2020;203:371–374. doi: 10.1164/rccm.202010-3823LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): A review. JAMA. 2020;324:782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 28.Chen N, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abate BB, Kassie AM, Kassaw MW, Aragie TG, Masresha SA. Sex difference in coronavirus disease (COVID-19): A systematic review and meta-analysis. BMJ Open. 2020;10:e040129. doi: 10.1136/bmjopen-2020-040129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karlberg J, Chong DS, Lai WY. Do men have a higher case fatality rate of severe acute respiratory syndrome than women do? Am. J. Epidemiol. 2004;159:229–231. doi: 10.1093/aje/kwh056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alghamdi IG, et al. The pattern of Middle East respiratory syndrome coronavirus in Saudi Arabia: A descriptive epidemiological analysis of data from the Saudi Ministry of Health. Int. J. Gen. Med. 2014;7:417–423. doi: 10.2147/IJGM.S67061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klang E, et al. Sex differences in age and comorbidities for COVID-19 mortality in urban New York City. SN Compr. Clin. Med. 2020;2:1319–1322. doi: 10.1007/s42399-020-00430-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y, et al. Remdesivir in adults with severe COVID-19: A randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yanez ND, Weiss NS, Romand JA, Treggiari MM. COVID-19 mortality risk for older men and women. BMC Public Health. 2020;20:1742. doi: 10.1186/s12889-020-09826-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu C, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou F, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Richardson S, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: A severity of disease classification system. Crit. Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 39.The L. The gendered dimensions of COVID-19. Lancet. 2020;395:1168. doi: 10.1016/S0140-6736(20)30823-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pradhan A, Olsson PE. Sex differences in severity and mortality from COVID-19: Are males more vulnerable? Biol. Sex Differ. 2020;11:53. doi: 10.1186/s13293-020-00330-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pivonello R, et al. Sex disparities in Covid-19 severity and outcome: Are men weaker or women stronger? Neuroendocrinology. 2020;111:1066–1085. doi: 10.1159/000513346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoffmann M, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao Y, et al. Single-cell RNA expression profiling of ACE2, the receptor of SARS-CoV-2. Am. J. Respir. Crit. Care Med. 2020;202:756–759. doi: 10.1164/rccm.202001-0179LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun M, et al. Sex differences in viral entry protein expression, host responses to SARS-CoV-2, and in vitro responses to sex steroid hormone treatment in COVID-19. Res Sq. 2020 doi: 10.21203/rs.3.rs-100914/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gross S, Jahn C, Cushman S, Bar C, Thum T. SARS-CoV-2 receptor ACE2-dependent implications on the cardiovascular system: From basic science to clinical implications. J. Mol. Cell Cardiol. 2020;144:47–53. doi: 10.1016/j.yjmcc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu K, et al. Factors associated with prolonged viral RNA shedding in patients with coronavirus disease 2019 (COVID-19) Clin. Infect. Dis. 2020;71:799–806. doi: 10.1093/cid/ciaa351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zheng S, et al. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: Retrospective cohort study. BMJ. 2020;369:m1443. doi: 10.1136/bmj.m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scully EP, Haverfield J, Ursin RL, Tannenbaum C, Klein SL. Considering how biological sex impacts immune responses and COVID-19 outcomes. Nat. Rev. Immunol. 2020;20:442–447. doi: 10.1038/s41577-020-0348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takahashi T, et al. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature. 2020;588:315–320. doi: 10.1038/s41586-020-2700-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takahashi T, Iwasaki A. Sex differences in immune responses. Science. 2021;371:347–348. doi: 10.1126/science.abe7199. [DOI] [PubMed] [Google Scholar]
- 51.Viveiros A, et al. Sex differences in COVID-19: Candidate pathways, genetics of ACE2, and sex hormones. Am. J. Physiol. Heart Circ. Physiol. 2021;320:H296–H304. doi: 10.1152/ajpheart.00755.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat. Med. 2011;30:377–399. doi: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- 53.Lee KJ, et al. Framework for the treatment and reporting of missing data in observational studies: The treatment and reporting of missing data in observational studies framework. J. Clin. Epidemiol. 2021;134:79–88. doi: 10.1016/j.jclinepi.2021.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kenward MG, Carpenter J. Multiple imputation: Current perspectives. Stat. Methods Med. Res. 2007;16:199–218. doi: 10.1177/0962280206075304. [DOI] [PubMed] [Google Scholar]
- 55.Carpenter JR, Smuk M. Missing data: A statistical framework for practice. Biom. J. 2021;63:915–947. doi: 10.1002/bimj.202000196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wunsch H, Linde-Zwirble WT, Angus DC. Methods to adjust for bias and confounding in critical care health services research involving observational data. J. Crit. Care. 2006;21:1–7. doi: 10.1016/j.jcrc.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 57.Groenwold RH, et al. Adjustment for continuous confounders: An example of how to prevent residual confounding. CMAJ. 2013;185:401–406. doi: 10.1503/cmaj.120592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Royston P, Altman DG, Sauerbrei W. Dichotomizing continuous predictors in multiple regression: A bad idea. Stat. Med. 2006;25:127–141. doi: 10.1002/sim.2331. [DOI] [PubMed] [Google Scholar]
- 59.Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among black patients and white patients with Covid-19. N. Engl. J. Med. 2020;382:2534–2543. doi: 10.1056/NEJMsa2011686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Williamson EJ, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Griffith GJ, et al. Collider bias undermines our understanding of COVID-19 disease risk and severity. Nat. Commun. 2020;11:5749. doi: 10.1038/s41467-020-19478-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Munafo MR, Tilling K, Taylor AE, Evans DM, Davey Smith G. Collider scope: When selection bias can substantially influence observed associations. Int. J. Epidemiol. 2018;47:226–235. doi: 10.1093/ije/dyx206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tas J, et al. Serial measurements in COVID-19-induced acute respiratory disease to unravel heterogeneity of the disease course: Design of the Maastricht Intensive Care COVID cohort (MaastrICCht) BMJ Open. 2020;10:e040175. doi: 10.1136/bmjopen-2020-040175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Prokop M, et al. CO-RADS: A categorical CT assessment scheme for patients suspected of having COVID-19-definition and evaluation. Radiology. 2020;296:E97–E104. doi: 10.1148/radiol.2020201473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Perkins NJ, et al. Principled approaches to missing data in epidemiologic studies. Am. J. Epidemiol. 2018;187:568–575. doi: 10.1093/aje/kwx348. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available due to data sharing agreements of the participating hospitals. Individual patient data and the pseudo-anonymized dataset will not be made available to others. Only data for the full cohort or a particular subcohort will be published and shared after the provision of a research proposal and signed data access agreement of each participating hospital.