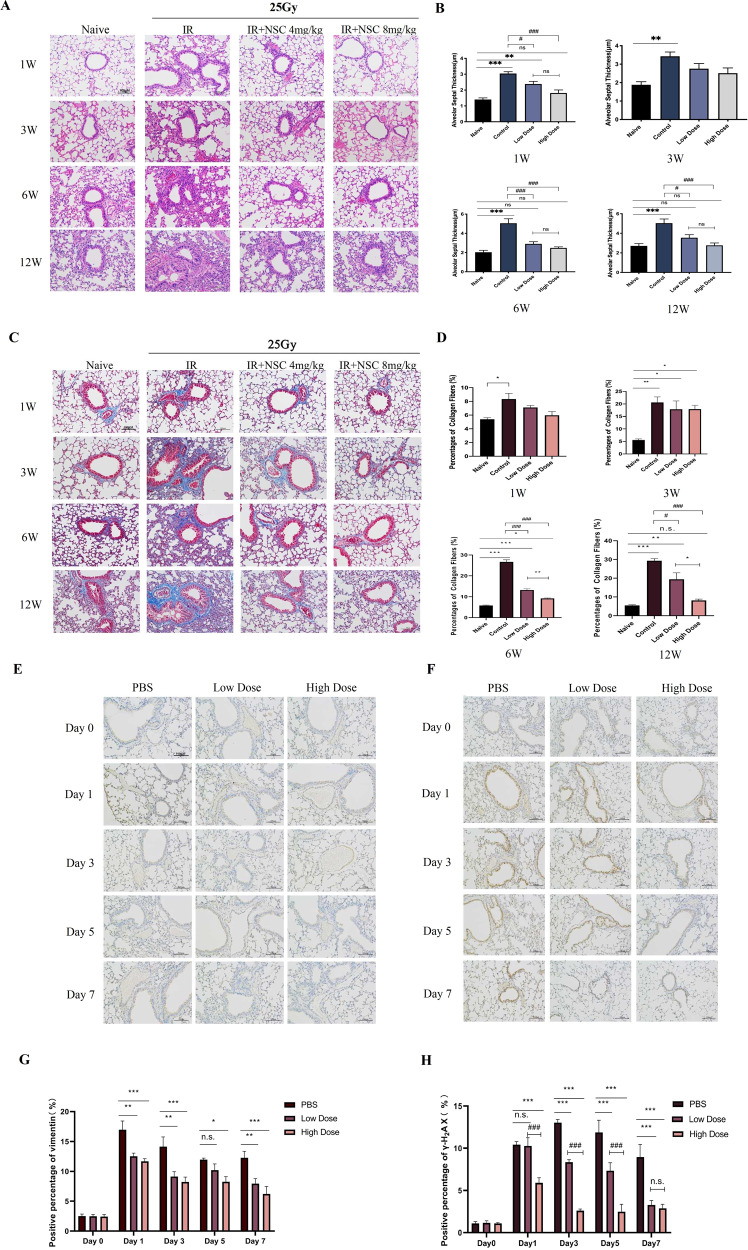
Fig. 1. Radiation-induced lung injury was alleviated by Rac1 inhibition.
C57BL/6 were randomly divided into four groups which were Naive (no IR + PBS), IR (IR + PBS), Low-Dose (IR + 4 mg/kg NSC23766) and High-Dose (IR + 8 mg/kg NSC23766) (N = 20). Rac1 inhibition was achieved by intraperitoneal injection of NSC23766, a specific Rac1 inhibitor. Intraperitoneal injection of PBS or NSC23766 was given once a day for a consecutive three days. 2 h after the 3rd injection, all mice except those of the Naive group were anesthetized and immobilized in a radiation-specific box for 25 Gy of local lung irradiation (IR). On the 1st, 3rd, 6th, and 12th week after IR, 5 mice from each group were sacrificed and lung tissues were collected. A, B Hematoxylin and eosin (H&E) staining and C, D Masson staining was conducted for the evaluation of pulmonary inflammation and fibrosis, respectively. E–H C57BL/6 mice were randomly divided into three groups, namely the PBS group (IR + PBS), the Low-Dose group (IR + 4 mg/kg NSC23766), and the High-Dose group (IR + 8 mg/kg NSC23766) (N = 15). Before radiation treatment (day 0) and on the 1st, 3rd, 5th, and 7th days after IR, lung tissues from 3 mice of each group were collected for immunohistochemistry analysis of vimentin and γ-H2AX, the important indicator of pulmonary epithelial–mesenchymal transformation (EMT) and DNA damage respectively. Image analysis was conducted using Image J software. ns represented that there was no statistically significant difference between the two groups. *, **, and *** represented P < 0.05, 0.01, and 0.001 between the corresponding groups, respectively. #, ##, and ### represented P < 0.05, 0.01, and 0.001 between the corresponding groups, respectively.