Abstract
Background
: We sought to assess the prevalence and impact of left ventricular thrombus (LVT) in patients with peripartum cardiomyopathy (PPCM).
Methods
: We performed a retrospective cohort study of all admissions with PPCM as the primary diagnosis from the Nationwide Inpatient Sample database over a 11-year period. Univariate analysis of all risk factors and outcomes and multivariable logistic regression analysis of certain variables were performed and represented as odds ratio (OR) with 95% confidence interval (CI). A p value of < 0.05 was considered statistically significant. Statistical analysis was performed using epiDisplay in ‘R’ studio.
Results
: In the time frame spanning 2005 -2014, 43,986 admissions with PPCM were found which included 43,534 without LVT and 452 patients with LVT. Black race was associated with a higher incidence of LV thrombus, (p value <0.001). Comorbidities more prevalent in the LVT group were smoking, drug abuse, pregnancy induced hypertension, diabetes with complications, valvular heart disease, connective tissue disorders, coagulopathy, anemia and depression. Adverse outcomes such as congestive heart failure, arrhythmias and stroke were higher in LVT group. Conversely, Caucasian race, obesity, preeclampsia (p <0.005) were higher in those without LVT. Mean length of stay (9 vs 5 days, p <0.001), in hospital mortality (3.32% vs 1.41%, p = 0.001) and mean hospitalization charges ($85,390 vs $48,033) were higher in those with LVT. However, on multivariate logistic regression, although stroke was higher in the LVT group (adjusted OR 5.51, 95% CI, 2.2, 13.81, 5.05, p 0.002), in-hospital mortality was not significantly different between the two groups (adjusted OR 1.17, 95% CI,0.32, 4.23, p = 0.817).
Conclusion
: Our study showed that PPCM patients with LV thrombus had worse outcomes with respect to stroke, length of stay and in hospital mortality. Higher prevalence in patients with black race, complicated diabetes, peripheral vascular disease, valvular disease, coagulopathy, smoking, drug abuse, depression and psychoses calls for special attention to such high-risk groups for aggressive risk factor modification.
Keywords: Peripartum cardiomyopathy; Left ventricular thrombus; Risk of stroke; In- hospital stay; Mortality; Smoking, obesity; Epidemiology
Abbreviations: PPCM, Peripartum Cardiomyopathy; LVT, Left Ventricular Thrombus; CI, Confidence Interval; LVEF, Left Ventricular Ejection Fraction; AHRQ, Agency for Healthcare Research and Quality; NIS, National Inpatient Sample; NYHA, New York Heart Association; ICD, International Classification of Diseases; LOS, Length of Stay; RHC, Right Heart Catheterization; LHC, Left Heart Catheterization
Graphical abstract
1. Introduction
The 2010 Heart Failure Association of the European Society of Cardiology defines Peripartum Cardiomyopathy (PPCM) as “an idiopathic cardiomyopathy presenting with Heart Failure (HF) secondary to left ventricular (LV) systolic dysfunction towards the end of pregnancy or in the months following delivery, where no other cause of heart failure is found”. The diagnostic criteria indicate that LV ejection fraction (LVEF) is < 45% and there may or may not be ventricular dilatation [1]. Data from the United States suggests the incidence of PPCM to be 1 in 968 live births [2]. Despite its significant prevalence and diagnosis, the exact pathophysiology of the condition remains unknown, but is likely thought to be multifactorial with recent emphasis on vasculo-hormonal hypothesis. The clinical presentation of patients with PPCM is highly variable ranging from NYHA class I-IV symptoms and cardiogenic shock to ventricular arrhythmias and cardiac arrest [1]. After stabilization and delivery, the mainstay of treatment is the usual guideline-directed medical therapy and device therapy for systolic heart failure [3,4]. However, an aspect where medical management of PPCM diverges from usual heart failure care and management of other forms of cardiomyopathies is the additional need for anticoagulation in these patients. It is a well-known fact that pregnancy is a hypercoagulable state and cardiac dilation, endothelial dysfunction and immobilization further contribute to it. Ongoing research has revealed that the burden of the PPCM is not only associated with the disease process itself but also with its potential complications including thrombo-embolism, arrhythmias and cardiogenic shock [5]. As per a recent study utilizing National Inpatient Sample database, thrombo-embolism is the most common complication of PPCM reported in 6.6% of cases [6]. The incidence of LV thrombus complicating PPCM in a single center study was reported to be as high as 17% [7]. Furthermore, several cases of left and right ventricular thrombi complicating peripartum cardiomyopathy have been reported [8], [9], [10]. These studies have shown favorable outcomes in these patients when appropriate treatment including anticoagulation is initiated in a timely manner. In such a scenario, it becomes imperative to study the differences in outcomes of peripartum cardiomyopathy patients with and without LV thrombus. Our study is a comparative analysis of demographics, risk factors, clinical course and outcomes of patients presenting with peripartum cardiomyopathy alone versus those with concomitant LV thrombus.
2. Methods
2.1. DATA source
We utilized the National inpatient sample (NIS) from Agency for Healthcare Research and Quality (AHRQ) from 2005 to 2014 [11]. Unweighted sample contains around 7 million observations, while the weighted sample contains 35 million records for each year. Weights were provided by NIS to calculate national estimates. As the database contains de-identified patient samples, it is deemed to be institutional board review exempt.
2.2. Inclusion and exclusion criteria
All patients with age ≥18 years, admitted to the hospital with the principal diagnosis of PPCM were included in the study. These patients were further divided into those with and without LV thrombus. We used ICD-9 Clinical Modification (CM) codes and ICD-9 procedure codes to retrieve patient samples and related procedures. The clinical classification software (CCS) developed by AHRQ was used to obtain codes for certain comorbidities. A detailed code summary is provided in supplementary Table 1. Patients with age <18 years with elective admission were excluded from the study. Patients with missing demographic data were excluded from the study [12].
2.3. Covariates
NIS data sample contains data regarding in-hospital outcomes, procedures, and other discharge-related information. Variables included patient characteristics, hospital demographics, and illness severity.
-
a
Patient Characteristics: Age, Race, Sex, Comorbidities, Disposition
-
b
Hospital Demographics: Location, Teaching status, Bed size, Region
-
c
Illness Severity: Length of stay (LOS), Mortality, Associated comorbidities
2.4. Study outcomes
Primary outcome of our study was the trend in incidence of PPCM and LV thrombus among hospitalized patients from 2005 to 2014. Secondary outcomes of our study were: (a) Comparison of hospital characteristics, patient characteristics with risk factors and complications among patients with PPCM complicated by LV thrombus versus PPCM alone, (b) Incidence of stroke, and (c) Impact of LV thrombus on in-hospital mortality, LOS, hospitalization cost and discharge disposition.
2.5. Statistical methods
Statistical analysis was performed with statistical software R 2.9.2 and epiDisplay package. The weighted sample was around 35 million discharges for each calendar year. Trends were calculated utilizing the trend weights provided with the data sample, and rates were expressed as the number of patients for that calendar year. Cochran-Mantel-Haenszel test was used to assess the significance of trends. Chi square test was used to compare categorical variables and linear regression was used to compare continuous variables such as age, LOS, hospitalization charge. Multivariate logistic regression analysis was used to analyze the effect of LV thrombus on in-hospital mortality and stroke. Multivariate analysis was adjusted for Age, race, hospital characteristics and comorbidities. Predictive margins were used to account for changing demographics, age with time, and impact on LOS. A two tailed p value of 0.05 was considered statistically significant.
3. Results
3.1. Primary outcome
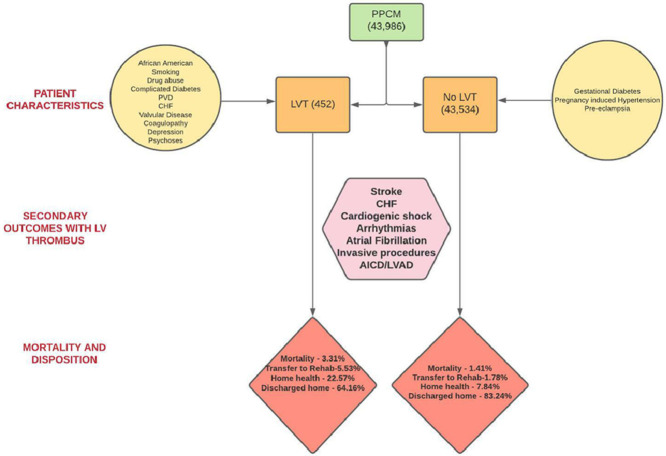
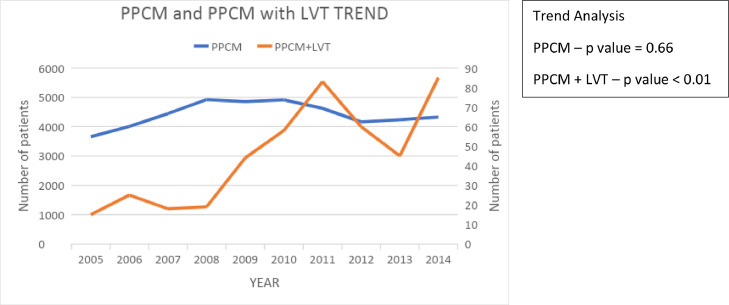
In our study period spanning 2005–2014, a total of 43,986 admissions with the primary diagnosis of peripartum cardiomyopathy were identified (Fig. 1), which included 43,534 admissions without LV thrombus and 452 with LV thrombus (1.03%). The incidence of PPCM remained relatively constant across the years, but the percentage of LV thrombus varied through the years with an overall increase from 2005 to 2014 (Fig. 2). The trend analysis had a p value of 0.66 for the PPCM group and < 0.01 for PPCM with LV thrombus group.
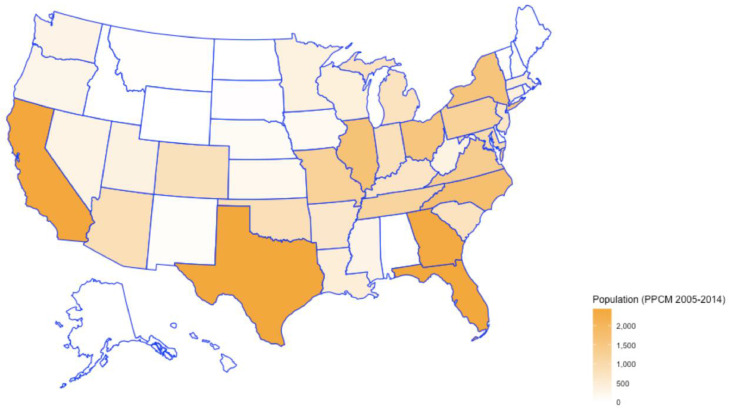
Fig. 1.
Distribution of PPCM patients across the US.
Fig. 2.
Trends of PPCM and PPCM + LVT over the study period. Left vertical represents number of patients for PPCM; Right vertical axis represents number of patients for PPCM with LVT.
3.2. Hospital demographics
A significant percentage of patients with LV thrombus complicating PPCM had presented to urban teaching (83.85%) and urban non-teaching (13.94%) hospitals, respectively, compared to a small proportion (2.22%), who had presented to rural or other settings. A similar trend was noted in those with PPCM alone with 62.03% diagnosed in urban teaching hospitals, 30.39% in urban non-teaching hospitals, and only 6.87% in rural settings. The larger bed size hospitals had a higher proportion of patients with LV thrombus (75%, compared to 68% in patients without LV thrombus, p value=0.002) (Table 1). Interestingly, but of unclear clinical significance, the majority of PPCM admissions with LV thrombus occurred in the months of July and December (12.17% of LV thrombus vs 8.03% of PPCM in July, p = 0.001 and another 12.17% in December vs 8.13%, p = 0.002).
Table 1.
Hospital characteristics.
| VARIABLES | PPCM with LVT | PPCM without LVT | p-Value |
|---|---|---|---|
| HOSPITAL REGION | |||
| Northeast | 88 (19.47%) | 6384 (14.67%) | <0.001 |
| Midwest | 94 (20.8%) | 9623 (22.1%) | 0.52 |
| South | 175 (38.72%) | 20,754 (47.67%) | <0.001 |
| West | 95 (21.02%) | 6773 (15.56%) | <0.001 |
| HOSPITAL BEDSIZE | |||
| Small | 26 (5.75%) | 3365 (7.72%) | 0.10 |
| Medium | 82 (18.14%) | 10,179(23.38%) | 0.01 |
| Large | 343 (75.88%) | 29,990 (68.88%) | <0.001 |
| HOSPITAL TEACHING STATUS | |||
| Not available | 5 (1.11%) | 310 (0.71%) | 0.33 |
| Rural | 5 (1.11%) | 2990 (6.87%) | <0.001 |
| Urban non-teaching | 63 (13.94) | 13,229 (30.39%) | <0.001 |
| Urban teaching | 379 (83.85%) | 27,005 (62.03%) | <0.001 |
3.3. Patient characteristics
The mean age of patients diagnosed with PPCM was 30 +/- 7 years, while that of PPCM patients complicated by LV thrombus was 29 +/- 7 years. Of these admissions, an equal percentage of White Americans (34.73%) and African Americans (34.9%) were diagnosed with PPCM. However, a greater percentage of African Americans were noted to have LV thrombus complicating the course (50.66% % vs. 28.76%). Majority of patients with LV thrombus (38.72%) were noted to belong to the Southern states [2].
The baseline comorbidities of the study cohort are summarized in (Table 2). The LV thrombus group had higher proportion of patients with smoking history (13.72% vs 5.5%; p value <0.001), diabetes with complications (3.54% vs1.49%, p value 0.001), congestive heart failure (CHF) (63.94% vs 50.27%, p value<0.001), valvular disease (19.47 vs 14.61, p value 0.004), depression (10.84% vs 7.20%, p value 0.003) and psychosis (3.32% vs 0.64%, p value,0.001). Conversely, PPCM patients without LV thrombus had higher percentage of gestational diabetes (6.79% vs 4.20%, p value 0.031), pregnancy induced hypertension (8.85% vs 33.33%, p value <0.001); and preeclampsia (11.44% vs 1.99%, p value <0.001) compared to those with LV thrombus. There was a significantly higher prevalence of drug abuse in the LV thrombus group (7.96% vs 4.75%, p value 0.002). More patients with LV thrombus had peripheral vascular disease and coagulopathy compared to those with PPCM alone.
Table 2.
Baseline characteristics of study cohort.
| PPCM with LVT (N = 452) | PPCM without LVT (N = 43,534) | p-Value | |
|---|---|---|---|
| SAMPLE SIZE (N) | 452 | 43,534 | |
| MEAN AGE (SD) YEARS | 29 (7) | 30 (7.1) | |
| FEMALE SEX (%) | 100 | 100 | |
| RACE | |||
| White | 130 (28.76%) | 15,118 (34.73%) | 0.01 |
| Black | 229 (50.66%) | 15,193 (34.9%) | <0.001 |
| Hispanic | 49 (10.84%) | 3822(8.87%) | 0.13 |
| Asian or Pacific Islander | 9 (1.99%) | 905 (2.08%) | 0.91 |
| Other | 24 (5.31%) | 2363 (5.43%) | 0.92 |
| Unavailable | 11 (2.44%) | 6133 (14.08) | <0.001 |
| ELIXHAUSER COMORBIDITIES | |||
| Smoking history | 62 (13.71%) | 2394 (5.49%) | <0.001 |
| Pregnancy related HTN | 40 (8.84%) | 14,510 (33.33%) | <0.001 |
| gestational diabetes | 19 (4.2%) | 2956 (6.79%) | 0.03 |
| preeclampsia | 9 (1.99%) | 4980 (11.43%) | <0.001 |
| Connective tissue disorders | 29 (6.41%) | 1981 (4.55%) | 0.06 |
| Cardiac congenital anomalies | 5 (1.1%) | 344 (0.79%) | 0.46 |
| Obesity | 55 (12.16%) | 7353 (16.89%) | 0.01 |
| Drug abuse | 36 (14.6%) | 2068 (4.75%) | <0.001 |
| Diabetes | 31 (6.85%) | 3030 (6.96%) | 0.94 |
| Diabetes with chronic complications | 16 (3.53%) | 649 (1.49%) | <0.001 |
| Hypertension | 135 (29.86%) | 14,327 (32.9%) | 0.17 |
| Hypothyroidism | 10 (2.21%) | 1881 (4.32%) | 0.03 |
| Liver disease | 5 (1.1%) | 327 (0.75%) | 0.40 |
| Renal failure | 20 (4.42%) | 1763 (4.04%) | 0.70 |
| Valvular disease | 88 (19.46%) | 6360 (14.6%) | <0.001 |
| Chronic pulmonary disease | 57 (12.61%) | 5472 (12.56%) | 0.98 |
| Deficiency anemias | 135 (29.86%) | 10,466 (24.04%) | <0.001 |
| Coagulopathy | 30 (6.63%) | 1828 (4.19%) | 0.01 |
| Depression | 49 (10.84%) | 3134 (7.19%) | <0.001 |
| Psychoses | 20 (4.42%) | 1245 (2.85%) | 0.05 |
| Paralysis | 15 (3.31%) | 279 (0.64%) | <0.001 |
3.4. Results of multivariate logistic regression analysis
In multivariate logistic regression analysis, smoking history, anemia and blood loss were independent predictors for the presence of LV thrombus in PPCM patients (Table 3). Black race was also an independent predictor of LV thrombus in multivariate logistic regression analysis (Supplementary Index Table S2).
Table 3.
Multivariate analysis of independent predictors of LVT.
| ELIXHAUSER COMORBIDITIES | 1MULTIVARIATE OR (95% CI) | p-Value |
|---|---|---|
| Smoking history | 2.22 (1.12–4.04) | 0.01 |
| Pregnancy related HTN | 0.21 (0.09–0.43) | <0.001 |
| Congestive heart failure | 1.86 (1.19–2.94) | 0.01 |
| Anemia | 2.05 (1.21–3.37) | 0.01 |
Adjusted for Age, race, hospital characteristics and comorbidities presented in Table 2.
3.5. Other secondary outcomes
The mean length of stay was higher in the cohort with LV thrombus (9 days vs 5 days, p value <0.001). There was significantly higher proportion of CHF (86% vs 61.52%, OR 3.86, 95% CI 2.9 - 5.04, p value 0.001), cardiogenic shock (11.73% vs 2.96%, OR 4.35, 95% CI: 3.25 - 5.82) arrhythmias (25.8% vs 19.32%, OR 1.45, 95% CI: 1.8 −3.4), and atrial fibrillation (4.20% vs 2.66%, OR 1.6; 95% CI 1.01 - 2.55, p value 0.045) in patients with LV thrombus. Patients with LV thrombus received significantly higher percentage of invasive procedures such as left heart catheterization (LHC) (4.42%), and 13.50% had either right heart catheterization (RHC) or both RHC and LHC (OR 3.2, 95% CI; 2.4 - 4.2, p value <0.001). On the other hand, in patients with PPCM alone, the numbers for LHC alone versus RHC or both RHC and LHC were lower at 2.53% and 4.63% respectively. More patients with LV thrombus required an implantable cardioverter defibrillator (ICD) (10.84% vs 6.50%: OR 1.74, 95 CI 1.29 −2.3, p value <0.001). A higher proportion of the LV thrombus cohort required left ventricular assist devices (LVAD) (2.21% vs 0.6%, OR 3.75; 95% CI 1.98 −7.10, p value < 0.001).
Stroke was significantly higher in the group with LV thrombus in both unadjusted (10.18% vs 1.24%, OR 9.0; 95% CI: 6.5 −12.3, p value <0.001) and adjusted analysis (OR 5.51, 95% CI: 2.2 - 13.81, p value 0.002).
Regarding in-hospital outcomes as depicted in Table 4, a significantly higher proportion of patients with LV thrombus needed transfer to another institution or needed home health care at discharge (p value <0.001). About 83.54% of patients with PPCM were discharged home with self-care, 7.84% required home health services and 1.78% were transferred to another institution for further care. For PPCM patients with LV thrombus, 64.16% could be discharged home with self-care, 22.57% required home health services and 5.53% required transfer to another institute for further care. The unadjusted in-hospital mortality rate was higher in patients with LV thrombus (3.32% vs 1.41%, OR 2.39; 95% CI 1.4 - 4.03; p value 0.001). However, the adjusted in-hospital mortality derived after adjusting for comorbidities, though numerically higher, was not statistically significant (OR 1.17, 95% CI 0.32, 4.23, p value 0.8). The mean hospitalization charges were higher in the LV thrombus group ($ 85,390 vs 48,033).
Table 4.
Outcomes.
| PPCM with LVT (N = 452) | PPCM without LVT (N = 43,534) | p-Value | |
|---|---|---|---|
| ADVERSE IN—HOSPITAL OUTCOMES | |||
| Congestive heart failure; non-hypertensive | 389 (86.06%) | 26,782 (61.51%) | <0.001 |
| Cardiogenic shock | 53 (11.72%) | 1289 (2.96%) | <0.001 |
| Cardiac dysrhythmias | 117 (25.88%) | 8411 (19.32%) | <0.001 |
| Atrial fibrillation | 19 (4.2%) | 1158 (2.65%) | 0.05 |
| Cardiac arrest | 14 (3.09%) | 814 (1.86%) | 0.06 |
| Left heart catheterization | 20 (4.42%) | 1101 (2.52%) | 0.01 |
| Combined Right and Left heart catheterization | 61 (13.49%) | 2016 (4.63%) | <0.001 |
| LVAD | 10 (2.21%) | 261 (0.59%) | <0.001 |
| Pulmonary edema | 12 (2.65%) | 801 (1.83%) | 0.21 |
| ICD placement | 49 (10.84%) | 2830 (6.5%) | <0.001 |
| DISPOSITION | |||
| Discharged home | 290 (64.16%) | 36,237 (83.24%) | <0.001 |
| Transferred to a short-term hospital for inpatient care | 15 (3.32%) | 1799 (4.13%) | 0.40 |
| Discharged to rehab | 25 (5.53%) | 774 (1.78%) | <0.001 |
| Home with home health care | 102 (22.57%) | 3414 (7.84%) | <0.001 |
| Left against medical advice | 5 (1.11%) | 697 (1.6%) | 0.41 |
| MEAN LOS (DAYS) | 9 | 5 | <0.001 |
| MEAN TOTAL CHARGE ($) | 85,390 $ | 48,033 $ | <0.001 |
| IN—HOSPITAL MORTALITY | 15 (3.31%) | 614 (1.41%) | <0.001 |
| 1 ADJ OR 1.17 (0.32 −4.23) | 0.8 | ||
| STROKE | 46 (10.17%) | 540 (1.24%) | <0.001 |
| 1 ADJ OR 5.52 (2.2, 13.81) | 0.002 | ||
Adjusted for Age, race, hospital characteristics and comorbidities presented in Table 2.
4. Discussion
An insight into the National Inpatient Sample database from 2005 to 2014 in our study revealed that 1.03% of patients with PPCM had concomitant LV thrombus complicating the clinical course. This percentage is lower in comparison to prior studies that were single center based or had a smaller population size [7,10]. Additionally, the percentage of patients with PPCM both with and without LV thrombus was significantly higher in urban hospitals compared to rural settings Table 1. Overall, this suggests an underestimation or underreporting of the true incidence of PPCM as well as LV thrombus, especially in rural settings, possibly due to lack of awareness, expertise, misdiagnosis or population demographics [2,8]. Furthermore, a review of prior studies on cardiac imaging has revealed that up to two-thirds of LV thrombi can be missed by the routinely performed non-contrast echocardiography (ECHO), with mural or small thrombi least likely to be detected [13].
Previous studies have provided data on the various risk factors that are associated with the development of PPCM, but one of the major limitations of these studies was the patient population size [14,15]. These risk factors included advanced maternal age, multiparity, gestational hypertension, preeclampsia and African American race. In our study, the mean age of women diagnosed with PPCM was 30 +/- 7 years and age of those with concomitant LV thrombus was 29 +/- 7 years, suggesting that advanced maternal age was associated with a higher incidence of both PPCM and LV thrombus. Recent National Center for Health Statistics (NCHS) data indicated an overall increase in the mean age for mothers from 2000 to 2014 for all birth orders, with the age at first birth having the highest increase from 24.9 years to 26.3 years [16]. This aids in explaining the overall increase in incidence of LV thrombus from 2005 to 2014, with variability through the years (Fig. 2). As identified in earlier studies [17], about one-third of patients with PPCM in our study had hypertension complicating pregnancy and childbirth and only 11.44% had been diagnosed with preeclampsia. However, these percentages were significantly lower at 8.85% and 1.99% respectively for the LV thrombus group. This suggests that although hypertension and preeclampsia may share similar pathophysiologies, preeclampsia does not necessarily lead to the development of peripartum cardiomyopathy [2]. The clinically significant association of LV thrombus with peripheral vascular disease and coagulopathy is also in accordance with these hypotheses. In our study, the African American race was associated with a higher incidence of LV thrombus in patients with PPCM. Majority of patients with LV thrombus (38.72%) were noted to belong to the Southern states, likely reflecting racial differences [2].
It is a well-known fact that social risk factors such as smoking and drug use are known to cause oxidative stress, vascular dysfunction and hypercoagulability [18]. This supports the higher incidence of LV thrombus in patients with significant history of smoking and drug use, which was consistent with the results obtained in our study [4,19]. Malnourishment had previously been linked as a risk factor for the development of PPCM [1] and obesity had been reported but not substantiated [1,2]. Similarly, previous studies had identified anemia (not necessarily classified as nutritional deficiency) in association with PPCM [17]. Our study revealed that 24.04% of patients with PPCM had an unspecified deficiency anemia and 23.71% had chronic blood loss anemia. On the contrary, 29.87% of those with LV thrombus had unspecified deficiency anemia, but only 8.85% had chronic blood loss anemia. This implies a stronger correlation of LV thrombus with acute pregnancy related nutritional deficiencies.
A higher percentage of patients in our analysis with concomitant LV thrombus and PPCM presented with acute congestive heart failure, cardiomyopathy, cardiac dysrhythmias, cardiogenic shock and atrial fibrillation compared to PPCM alone. This implies a more severe and complicated presentation of those with LV thrombus, further necessitating the need for increased awareness of thrombo-embolic and other complications of PPCM. This is imperative for early diagnosis and aggressive therapy in these patients as an attempt to prevent complications and improve mortality [20].
The clinical course was complicated by stroke in all PPCM patients. However, the incidence was higher in PPCM patients with concomitant LV thrombus vs those with PPCM alone (3.54% and 0.40% respectively). The same was also true for other cerebrovascular accidents (10.18% vs 1.24% respectively). Previously, there have been case reports of patients with PPCM presenting with massive cerebral or pulmonary embolism [8,21,22]. This reiterates the fact that PPCM is associated with higher rates of thrombo-embolism as compared to other cardiomyopathies and warrants a need for anticoagulation for prevention of such complications [2]. A higher percentage of patients with concomitant LV thrombus with PPCM vs PPCM alone (10.84% vs 6.50%) developed persistent heart failure requiring ICD placement.
PPCM is relatively uncommon but remains an important cardiac cause of maternal morbidity and mortality in previously healthy women [23]. Finally, in our study, the unadjusted in-hospital mortality rate was higher in patients with LV thrombus complicating PPCM, emphasizing the need for strong suspicion, early diagnosis and institution of guideline directed medical therapy in these patients (3.32% vs 1.41%, OR 2.4, p 0.001). Higher number of patients belonging to PPCM with concomitant LV thrombus group needed home health care services or transfer to long term care facilities than those without, suggesting more morbidity and debility with a longer route to recovery. The presence of LV thrombus has independently been shown to be associated with lack of recovery of LVEF in PPCM patients [7]. Hence, these patients require higher levels of care for a longer period, sometimes only leading to partial recovery contributing to the significant health care burden of the illness. Prolonged illness can lead to increased incidence of depression in these patients and possibly lead to difficulties in treatment compliance and follow up [24].
5. Limitations
The retrospective observational study design and administrative data have their inherent limitations. As hospitalizations and not individual patients are represented in the data, there is a potential for overestimation of the number of patients, especially, if some of the patients had readmissions during the same pregnancy or had more than one pregnancy with similar diagnosis. Although the mean age of patients in the study subset was ∼ 30+/- 7, some of the older patients could have had comorbidities such as diabetes and hypertension which could have independently caused heart failure. The number of PPCM patients with LV thrombus in our study cohort was much smaller compared to those without LV thrombus, which could have potentially affected the significance of certain comparisons due to low power. There are no studies that have studied the validity of ICD- 9 codes used for PPCM and LV thrombus, however, these codes have been used by several investigators in the past to accurately identify PPCM patients from the HCUP database [25,26]. Study results can only state higher association with stroke in patients with LV thrombus but cannot prove causality. Hemodynamic data such as vital signs, laboratory data and imaging parameters are not available in this data. Furthermore, information on medications and their compliance, readmissions and long-term follow-up is not available. However, most of the limitations are attenuated by the considerable sample size obtained from these large databases.
6. Conclusion
Our study showed that PPCM patients with LV thrombus had worse outcomes with respect to stroke, length of stay and in hospital mortality. Higher prevalence in patients with black race, complicated diabetes, peripheral vascular disease, valvular disease, coagulopathy, smoking, drug abuse, depression and psychoses calls for special attention to such high-risk groups for aggressive risk factor modification.
Disclosures
There are no relationships with industry nor other conflicts of interest.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ajpc.2021.100313.
Contributor Information
Kritika Luthra, Email: kluthra@mercy.com.
Sindhu R. Avula, Email: savula@kumc.edu.
Murugesan Raju, Email: rajum@health.missouri.edu.
Karthik Gangu, Email: karthik.gangu@health.missouri.edu.
Zainulabedin Waqar, Email: zawaqar@mercy.com.
Rajiv Doddamani, Email: rajivdoddamani@hotmail.com.
Bhanu Harshita Settipalle, Email: bhanuharshita@gmail.com.
Jay Shah, Email: jjshah@mercy.com.
Syed Sohail Ali, Email: drsohailali@gmail.com.
Hemindermeet Singh, Email: drhemindermeet.94@gmail.com.
Appendix. Supplementary materials
References
- 1.Sliwa K., Hilfiker-Kleiner D., Petrie M.C., et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of peripartum cardiomyopathy: a position statement from the Heart Failure Association of the European Society of Cardiology Working Group on peripartum cardiomyopathy. Eur J Heart Fail. 2010;12:767–778. doi: 10.1093/eurjhf/hfq120. [DOI] [PubMed] [Google Scholar]
- 2.Arany Z., Elkayam U. Peripartum Cardiomyopathy. Circulation. 2016;133:1397–1409. doi: 10.1161/CIRCULATIONAHA.115.020491. [DOI] [PubMed] [Google Scholar]
- 3.Regitz-Zagrosek V., Roos-Hesselink J.W., Bauersachs J., et al. 2018 ESC Guidelines for the management of cardiovascular diseases during pregnancy. Kardiol Pol. 2019;77:245–326. doi: 10.5603/KP.2019.0049. [DOI] [PubMed] [Google Scholar]
- 4.Cowgill J.A., Francis S.A., Sawyer D.B. Anthracycline and peripartum cardiomyopathies. Circ Res. 2019;124:1633–1646. doi: 10.1161/CIRCRESAHA.119.313577. [DOI] [PubMed] [Google Scholar]
- 5.Hoevelmann J., Muller E., Hohlfeld A., et al. Outcomes and complications of peripartum cardiomyopathy: protocol for a systematic review and meta-analysis. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2021-054994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kolte D., Khera S., Aronow W.S., et al. Temporal Trends in Incidence and Outcomes of Peripartum Cardiomyopathy in the United States: a Nationwide Population-Based Study. J Am Heart Assoc. 2014;3 doi: 10.1161/jaha.114.001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amos A.M., Jaber W.A., Russell S.D. Improved outcomes in peripartum cardiomyopathy with contemporary. Am Heart J. 2006;152:509–513. doi: 10.1016/j.ahj.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 8.Sakamoto A., Hosoya N., Kageyama S., et al. Peripartum cardiomyopathy with biventricular thrombus which led to massive cerebral embolism. J Cardiol Cases. 2014;9:71–74. doi: 10.1016/j.jccase.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Damorou F.J., Kane A., Napporn G., et al. Biventricular thrombus complicating peripartum cardiomyopathy. A case report. Dakar Med. 2000;45:199–201. [PubMed] [Google Scholar]
- 10.Napporn A.G., Kane A., Damorou J.M., et al. Intraventricular thrombosis complicating peri-partum idiopathic myocardiopathy. Ann Cardiol Angeiol. 2000;49:309–314. [PubMed] [Google Scholar]
- 11.NIS Database Documentation Archive. https://www.hcup-us.ahrq.gov/db/nation/nis/nisarchive.jsp (accessed 7 Nov 2021).
- 12.NIS Database Documentation Archive. https://www.hcup-us.ahrq.gov/db/nation/nis/nisarchive.jsp (accessed 7 Nov 2021).
- 13.Chinitz J.S., Mendoza D.D., Kim R.J., et al. Cardiac imaging for assessment of left ventricular thrombus. US Cardiol Rev. 2009;6:27–33. doi: 10.15420/usc.2009.6.2.27. [DOI] [Google Scholar]
- 14.Pearson G.D., Veille J.C., Rahimtoola S., et al. Peripartum cardiomyopathy: national Heart, Lung, and Blood Institute and Office of Rare Diseases (National Institutes of Health) workshop recommendations and review. JAMA. 2000;283:1183–1188. doi: 10.1001/jama.283.9.1183. [DOI] [PubMed] [Google Scholar]
- 15.Whitehead S.J., Berg C.J., Chang J. Pregnancy-related mortality due to cardiomyopathy: United States, 1991-1997. Obstet Gynecol. 2003;102:1326–1331. doi: 10.1016/j.obstetgynecol.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 16.Mathews T.J., Hamilton B.E. Mean age of mothers is on the rise: United States, 2000-2014. NCHS Data Brief. 2016:1–8. [PubMed] [Google Scholar]
- 17.Kao D.P., Hsich E., Lindenfeld J. Characteristics, adverse events, and racial differences among delivering mothers with peripartum cardiomyopathy. JACC Heart Fail. 2013;1:409–416. doi: 10.1016/j.jchf.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carnevale R., Cammisotto V., Pagano F., et al. Effects of smoking on oxidative stress and vascular function. Smok Prevent Cessat. 2018 doi: 10.5772/intechopen.78319. [DOI] [Google Scholar]
- 19.Bello N.A., Arany Z. Molecular mechanisms of peripartum cardiomyopathy: a vascular/hormonal hypothesis. Trends Cardiovasc Med. 2015;25:499–504. doi: 10.1016/j.tcm.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goland S., Modi K., Bitar F., et al. Clinical profile and predictors of complications in peripartum cardiomyopathy. J Card Fail. 2009;15:645–650. doi: 10.1016/j.cardfail.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 21.Koc M. Development of biventricular large apical thrombi and cerebral embolism in a young woman with peripartum cardiomyopathy. Arch Turk Soc Cardiol. 2011;39:591–594. doi: 10.5543/tkda.2011.01534. [DOI] [PubMed] [Google Scholar]
- 22.Gunes H. Biventricular thrombi and pulmonary embolism in a young woman with peripartum cardiomyopathy. J Clin Anal Med. 2018;9 doi: 10.4328/jcam.5749. [DOI] [Google Scholar]
- 23.Goland S., Elkayam U. Peripartum cardiomyopathy: approach to management. Curr Opin Cardiol. 2018;33:347–353. doi: 10.1097/HCO.0000000000000516. [DOI] [PubMed] [Google Scholar]
- 24.Rosman L., Salmoirago-Blotcher E., Cahill J., et al. Depression and health behaviors in women with Peripartum Cardiomyopathy. Heart Lung. 2017;46:363–368. doi: 10.1016/j.hrtlng.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 25.Kolte D., Khera S., Aronow W.S., et al. Temporal trends in incidence and outcomes of peripartum cardiomyopathy in the United States: a nationwide population-based study. J Am Heart Assoc. 2014;3 doi: 10.1161/JAHA.114.001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ram P., Shah M., Sirinvaravong N., et al. Left ventricular thrombosis in acute anterior myocardial infarction: evaluation of hospital mortality, thromboembolism, and bleeding. Clin Cardiol. 2018;41:1289–1296. doi: 10.1002/clc.23039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.