1. Introduction
While acute nociceptive pain is necessary for survival, pain that persists after an injury has healed highlights a malfunction of the sensory system [51]. Pain stimuli are conveyed toward the central nervous system through nociceptive fibers (C and Aδ) innervating the superficial layers of the spinal dorsal horn (lamina I and II) before reaching the brain. The substantia gelatinosa (SG, lamina I and II) constitutes the first site of synaptic integration in pain pathways [21; 54], where central terminals of primary afferent neurons and spinal second order neurons form the first sensory synapse, a critical hub in the transmission and regulation of ascending pain pathways [56]. Central sensitization, a fundamental component of pathological pain, is due to an increased efficacy in neurotransmission and synaptic plasticity in the dorsal horn [28; 29; 64]. It relies on a variety of ion channels expressed all along the afferent nociceptive fibers [57]. In particular, the voltage-gated sodium channel Nav1.7 and the voltage-gated calcium channel Cav2.2 have a dominant role in controlling the initiation of the signal and the release of neurotransmitters in the superficial dorsal horn, respectively [6; 26].
A dual regulator of Nav1.7 and Cav2.2 channels is the collapsin response mediator protein 2 (CRMP2) [3; 7], first identified as an important modulator of axonal growth by binding to tubulin dimers [18; 20; 23] and subsequently reported to be involved in neuroprotection through regulation of NMDAR/NCX3 [2; 41; 58]. Through concerted actions of post-translational modifications, CRMP2 interacts with Nav1.7 and Cav2.2 to promote their expression at the membrane [15; 38]; preventing addition of the post-translational modifications leads to their internalization [15; 17; 48]. This reversible action of CRMP2 makes it as a key modulator of electrogenesis and neurotransmitter release at nociceptive synapses. Additionally, we and others have shown that phosphorylation of CRMP2 by cyclin dependent kinase 5 (Cdk5) regulates spinal nociceptive neurotransmission [59; 61] and that CRMP2 phosphorylation is increased following an injury [42], while blocking SUMOylation of CRMP2 reverses neuropathic pain [38; 40]. However, the role of CRMP2 in aberrant excitatory synaptic transmission underlying neuropathic pain processing after peripheral nerve injury was never investigated. Availability of a transgenic mouse homozygous for the floxed Crmp2 allele (CRMP2f/f) [43] enables selective Cre-recombinase-mediated knockout of CRMP2 expression at the spinal gate for nociceptive neurotransmission and allows us to address this question.
Here, we used slice electrophysiology combined with cell-specific conditional knockout of CRMP2 from CRMP2f/f mice, in parallel with siRNA-mediated knockout of CRMP2 in rats, to show that neuronal CRMP2 exclusively regulates excitatory spinal neurotransmission. To further elucidate the in vivo consequence of the conditional knockout of CRMP2 in CRMP2f/f mice, we used the well-known spared nerve injury (SNI) model of neuropathic pain and demonstrated pain resilience in male and female floxed CRMP2f/f mice, as inferred from their refractoriness to develop persistent mechanical allodynia. Our data strongly suggests that CRMP2 is a key regulator of glutamatergic sensory neurotransmission that contributes to the initiation and maintenance of chronic neuropathic pain.
2. Materials and Methods
2.1. Animals
Pathogen-free, male Sprague-Dawley rat pups (postnatal 10–15 days; Envigo) and pups (8–15 days old) from male mice homozygous for the floxed Crmp2 allele (CRMP2f/f) mouse [43] were used for electrophysiological experiments. Adult male (21–28g) and female (16–20g) CRMP2f/f mice were used for behavioral experiments. All animals were housed in the University of Arizona Laboratory Animal Research Center in temperature-controlled (23 ± 3°C) and light-controlled (12-hour light/12-hour dark cycle; lights on 7:00–19:00) rooms with standard rodent chow and water available ad libitum. Mice were housed in groups of 4 to 5. During breeding and until weaning of the pups, CRMP2f/f mice were fed 3 times weekly with Love Mash Rodent Reproductive diet (Cat# S3823P; Bio-Serv, Flemington, NJ) to improve fertility for both males and females, increase the litter size, and increase pup survival rate. The Institutional Animal Care and Use Committee of the College of Medicine at the University of Arizona approved all experiments. All procedures were conducted in accordance with the Guide for Care and Use of Laboratory Animals published by the National Institute of Health. All efforts were made to minimize animal suffering to reduce the number of the animals used. Animals were randomly assigned to treatment or control groups for all experiments. All electrophysiology and behavioral experiments were performed by experimenters who were blinded to the treatment groups.
2.2. Reagents
All chemicals, unless noted, were purchased from Sigma (St Louis, MO). As reported by us earlier [45], CRMP2 siRNA (5′-GTAAACTCCTTCCTCGTGT-3′) [3] or siRNA Control (Cat# 12935300) were obtained from Thermo Fisher Scientific, Waltham, MA. siRNAs were diluted to 6 μM in 5μl sterile glucose solution. Then Turbofect in vivo transfection reagent (Cat# R0541, Thermo Fisher Scientific, Waltham, MA) was added at 1/17 dilution. AAV9.CaMKIIα.GFP-Cre (Cat# 105551-AAV9), AAV9.CaMKII.eGFP (Cat#105541-AAV9), AAV5.GFAP.Cre (Cat# 105550-AAV5) and AAV.GFAP.eGFP (Cat# 105549-AAV5) were obtained from Addgene (Watertown, MA). Validated antibodies were purchased as follows: anti-CRMP2 polyclonal antibody (Sigma-Aldrich, Cat# C2993) and anti-βIII-tubulin (Cat# G712A, Promega).
2.3. Intrathecal injections (lumbar puncture)
Young (P12–15) rats and mice or adult mice (females: 15–20g, males: 21–28g) were deeply anesthetized with isoflurane (4% for induction and 2% for maintaining). The lower half of the animal’s back was shaved. A 25G needle was introduced, perpendicular to the surface through the widest L4-L5 intervertebral level and lowered until it met the vertebral body. Occasionally, a quick flicking of the tail could be observed and served as a proxy for optimal placement of the needle. The moment of penetration into the intrathecal space could be noticed by a change in resistance to the introduction of the needle. Young rats (P10–15) were injected with 10 μL of indicated siRNA depending on their treatment group. Young (P8–15) and adult mice were injected with 5 μL and 20 μL of the indicated virus (Titer≥ 1×1013 viral genomes/mL) depending on their treatment group, respectively. The experiments (electrophysiology or behavior) were performed two days following the intrathecal injections to allow siRNA- or viral-mediated deletion.
2.4. Preparation of Spinal Cord Slices
Rats or mice were deeply anesthetized with isoflurane (4% for induction and 2% for maintaining). For spinal nerve block, 0.3 mL of 2% lidocaine was injected to both sides of L4 to L5 lumbar vertebrae. Laminectomy (Fig. 1A) was performed from mid-thoracic to low lumbar levels, and the spinal cord was quickly removed to cold modified ACSF oxygenated with 95% O2 and 5% CO2. The ACSF for both rat and mouse dissection contained the following (in millimolar): 80 NaCl, 2.5 KCl, 1.25 NaH2PO4, 0.5 CaCl2.2H2O, 3.5 MgCl2.6H2O, 25 NaHCO3, 75 Sucrose, 1.3 ascorbate, 3.0 sodium pyruvate with pH at 7.4 and osmolarity at 310 mOsm. Transverse 400 μm- (rats) or 360 μm- (mice) thick slices were obtained by a vibratome (VT1200S; Leica, Nussloch, Germany). Slices were then incubated for 45 mins (rats) or 30 mins (mice) at 37°C before a 1h incubation at RT in an oxygenated recording solution containing the following (in millimolar): 125 NaCl, 2.5 KCl, 1.25 NaH2PO4, 2 CaCl2.2H2O, 1 MgCl2.6H2O, 26 NaHCO3, 25 D-Glucose, 1.3 ascorbate, 3.0 sodium pyruvate with pH at 7.4 and osmolarity at 320 mOsm. The slices were then positioned in a recording chamber and continuously perfused with oxygenated recording solution at a rate of 3 to 4 mL/min before electrophysiological recordings at room temperature (RT).
Fig 1. siRNA-mediated knockdown of CRMP2 decreases the frequency and amplitude of spontaneous excitatory postsynaptic currents (sEPSCs) in the Substantia gelatinosa region of the lumbar dorsal horn.
A. Schematic representation of the method used for slice electrophysiology and the analysis of CRMP2 expression in the DRGs and SCs of rat pups. Two days following intrathecal injections, DRGs and SCs of a first cohort of each treatment group were removed for western blot analyses, while spinal cords of a second cohort of each treatment group were dissected for slice recordings. B. Representative immunoblots showing the expression of CRMP2 in the DRG and the SDH of rats from each treatment group. Samples were harvested 2 days following injections. βIII-Tubulin is used as a loading control. C. Bar graph with scatter plot showing decreased CRMP2 expression in DRG and SDH of rats injected with the siRNA targeting CRMP2 (CRMP2 siRNA) compared to control (n=3 per condition). D. Representative traces of sEPSC recordings from substantia gelatinosa (SG) neurons transfected with control siRNA or siRNA targeting CRMP2 (CRMP2 siRNA). E. Cumulative distribution of sEPSC inter-event intervals recorded from SG neurons transfected with CRMP2 siRNA revealed a rightward shift towards longer inter-event intervals compared to control. Inset: Bar graph with scatter plot showing decreased sEPSC frequency compared to control. F. A cumulative distribution of sEPSC amplitudes revealed a rightward shift toward smaller amplitudes for neurons transfected with CRMP2 siRNA. Inset: Bar graph with scatter plot showing decreased sEPSC amplitude compared to control. Data are expressed as means ± SEM. Unpaired t-test with Welch’s. For full statistical analyses, see Table 1. sEPSC: spontaneous Excitatory Postsynaptic Currents, SG: Substantia Gelatinosa, DRG: Dorsal Root Ganglion, SC: Spinal Cord, SDH: Spinal Dorsal Horn.
2.5. Electrophysiological recordings in spinal cord slices by whole-cell patch-clamp
Substantia Gelatinosa neurons (lamina I/IIa) were visualized and identified in the slices by means of infrared differential interferences contrast video microscopy on an upright microscope (FN1; Nikon, Tokyo, Japan) equipped with a 3.40/0.80 water-immersion objective and a charged-coupled device camera. Patch pipettes with resistance at 6 to 10 MΩ were made from borosilicate glass (Sutter Instruments, Novato, CA) on a four-step micropipette puller (P-90; Sutter Instruments, Novato, CA). For spontaneous and miniature excitatory postsynaptic current (sEPSC and mEPSC) recordings the pipette solution contained the following (in millimolar): 120 potassium-gluconate, 20 KCl, 2 MgCl2.6H2O, 2.0 Na2-ATP, 0.5 Na-GTP, 20 HEPES, 0.5 EGTA with pH at 7.4 and osmolarity at 310 mOsm. For spontaneous and miniature inhibitory postsynaptic current (sIPSC and mIPSC) recordings, the pipette solution contained the following (in millimolar): 140 CsCl, 2 MgCl2.6H2O, 1.0 CaCl2.2H2O, 1.0 EGTA, 10 HEPES, 5.0 K-ATP, 0.1 Na-GTP with pH at 7.4 and osmolarity at 310 mOsm. For all recordings, the membrane potential was held at −60 mV using a PATCHMASTER software in combination with a patch clamp amplifier (EPC10; HEKA Elektronik, Lambrecht, Germany).
The whole-cell configuration was obtained in voltage-clamp mode. To record sEPSCs, bicuculline methiodide (10 μM, Cat# 14343, Sigma Aldrich) and strychnine (2 μM, Cat# S0532, Sigma Aldrich) were added to the recording solution to block γ-aminobutyric acid-activated (GABA) and glycine-activated currents. To record sIPSCs, 6-cyano-7-nitroquinoxaline-2,3-dioneis (CNQX; 20μM, Cat# C239, Sigma Aldrich) and DAP-V (50 μM, Cat# AB120003, Abcam) were added to the recording solution to block α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid- activated (AMPA) and N-methyl-D-aspartate-activated (NMDA) currents. Tetrodotoxin (TTX; 1 μM, Cat# ab120054, Abcam) was added to block action potentials when we were recording mEPSCs and mIPSCs. Hyperpolarizing step pulses (5 mV for 50 milliseconds) were periodically delivered to monitor the access resistance (15–25 MΩ), and recordings were discontinued if the access resistance changed by more than 20%. For each neuron, sEPSCs, sIPSCs, mEPSCs or mIPSCs were recorded for a total duration of 2 minutes. Currents were filtered at 3kHz and digitized at 5 kHz. Data were further analyzed by the Mini-Analysis Program (Synatosoft Inc., NJ) to provide spreadsheets for the generation of cumulative probability plots. The frequency and amplitude of the recordings were compared between neurons from animals in control and the indicated groups. As the frequency of postsynaptic currents is influenced by the temporal summation of the currents on the presynaptic terminal of the synapse, it follows that any change of the frequency suggests a modification occurring on the presynaptic component of the synapse (including the addition of afferent elements, a modification of the fiber, a delay in the action potential). In parallel, the amplitude of postsynaptic currents is tied to the reuptake of neurotransmitters by receptors on the postsynaptic part of the synapse. Consequently, any change in the amplitude translates into a modification occurring at the postsynaptic side of the terminals (including a decrease or an increase of the expression of the receptors at the membrane) [32; 33; 53]. For each experiment, GFP fluorescence was used to control for successful expression of the Cre recombinase in the slices.
2.6. Hot plate test
CRMP2f/f mice were placed on a metal plate (Stoelting, Wood Dale, IL) warmed to 52°C and a timer was started. Latency to the first response (flinching or licking the hind paws or jumping) was recorded. Cutoff times were used to prevent tissue damage (20 seconds), and any mouse that reached the cutoff time was assigned the value of the cutoff time as their latency time.
2.7. Tail-flick test
The distal third of the tail of CRMP2f/f mice was immersed in warm water (52°C). Latency to remove the tail from the water (tail-flick latency) was recorded. To prevent tissue damage, a 10-second cutoff was used and animals that did not react before the cutoff were attributed a latency of 10 seconds.
2.8. Spared Nerve Injury model of neuropathic pain
To prepare the neuropathic pain model induced by spared nerve injury (SNI), we transected the common peroneal and tibial branches of the right sciatic nerve with ~1 mm of the nerve removed and left the sural nerve intact. Animals were tested on day 15 after the surgery, at which time they had developed stable and maximal mechanical allodynia. CRMP2f/f mice were habituated in plastic chambers on a mesh floor. Calibrated von Frey filaments with sequentially increasing spring coefficients were applied to the hind paw of each mouse, which allowed for the consistent application of constant force stimuli. One filament was applied 5 times in a round of testing. The filament force evoking paw withdrawal more than 3 times in a round of testing was defined as the mechanical threshold. The cutoff threshold was 4 g. The von Frey test was performed on the lateral part of the right plantar surface where the sural nerve innervates the hind paw.
2.9. Immunoblot preparation and analysis
Samples (DRG and Spinal cord) were harvested two days after intrathecal injections (Fig. 1A). Protein concentrations were determined using the BCA protein assay (Cat# PI23225; Thermo Fisher Scientific). Indicated samples were loaded on 4% to 20% Novex gels (Cat# EC60285BOX; Thermo Fisher Scientific). Proteins were transferred for 1 hour at 120 V using TGS (25 mM Tris pH = 8.5, 192 mM glycine, 0.1% (mass/vol) SDS), 20% (vol/vol) methanol as transfer buffer to polyvinylidene difluoride membranes 0.45 μm (Cat# IPVH00010); Millipore, Billerica, MA), preactivated in pure methanol. After transfer, the membranes were blocked at RT for 1 hour with TBST (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.1% tween 20), 5% (mass/vol) nonfat dry milk, then incubated separately in the primary antibodies CRMP2 (Cat# C2993, Sigma, St. Louis, MO), and βIII-Tubulin (Cat# G712A, Promega) in TBST, 5% (mass/vol) BSA, overnight at 4°C. After incubation in horseradish peroxidase-conjugated secondary antibodies from Jackson ImmunoResearch, blots were revealed by enhanced luminescence (WBKLS0500; Millipore) before exposure to photographic film. Films were scanned, digitized, and quantified using Un-Scan-It gel version 7.1 scanning software by Silk Scientific Inc.
2.10. Statistical Analysis
Statistical analyses were performed using GraphPad Prism 8 (GraphPad, San Diego, CA). All data were first tested or a Gaussian distribution using a D’Agostino-Pearson test. The statistical significance between means was determined by either parametric or non-parametric Student’s t-test, analysis of variance (ANOVA) followed by post hoc comparisons (Tukey, Sidak) using Prism 8. Statistical significance was set at α < 0.05. Error bars in the graphs represent mean ± SEM. See statistical analyses described in Table 1. All data were plotted in Prism 8.
Table 1.
Statistical analyses of experiments
| Figure panel | Assay | Statistical test; findings | Post hoc analysis (adjusted P-values) | No. of subjects |
|---|---|---|---|---|
| Fig 1B and 1C | CRMP2 expression in male rats – Western blot |
t-test with Welch’s correction DRG: P < 0.0001 SDH: P < 0.0001 |
Control siRNA males: n = 3 CRMP2 siRNA males: n = 3 |
|
| Fig 1E and 1F | Slice electrophysiology – sEPSC recordings |
t-test with Welch’s correction Frequency: P = 0.0005 Amplitude: P = 0.0033 |
Control siRNA males: n = 3 (14 cells) CRMP2 siRNA males: n = 3 (20 cells) |
|
| Fig 2B and 2C | Slice electrophysiology – sIPSC recordings | Mann-Whitney test Frequency: P = 0.8710 Amplitude: P = 0.5546 |
Control siRNA males: n = 4 (18 cells) CRMP2 siRNA males: n = 4 (23 cells) |
|
| Fig 3B and 3C | Slice electrophysiology – mEPSC recordings |
t-test with Welch’s correction Frequency: P = 0.4709 Amplitude: P = 0.6025 |
Control siRNA males: n = 3 (17 cells) CRMP2 siRNA males: n = 3 (12 cells) |
|
| Fig 3E and 3F | Slice electrophysiology – mIPSC recordings |
t-test with Welch’s correction Frequency: P = 0.1438 Amplitude: P = 0.0554 |
Control siRNA males: n = 4 (16 cells) CRMP2 siRNA males: n = 4 (16 cells) |
|
| Fig 4B | CRMP2 expression in CaMKIIf/f mice – Western blot |
t-test with Welch’s correction DRG: P = 0.0065 SDH: P = 0.00007 |
CRMP2f/f Control: n = 3 CRMP2f/f CaMKII Cre: n = 3 |
|
| Fig 5B and 4C | Slice electrophysiology – sEPSC recordings |
t-test with Welch’s correction Frequency: P = 0.0014 Amplitude: P = 0.0020 |
CRMP2f/f Control males: n = 2 (13 cells) CRMP2f/f CaMKII Cre males: n = 3 (13 cells) |
|
| Fig 5E and 4F | Slice electrophysiology – mEPSC recordings |
t-test with Welch’s correction Frequency: P = 0.4324 Amplitude: P = 0.4384 |
CRMP2f/f Control males: n = 2 (12 cells) CRMP2f/f CaMKII Cre males: n = 3 (13 cells) |
|
| Fig 6B and 5C | Slice electrophysiology – sEPSC recordings | Mann-Whitney test Frequency: P = 0.5118 Amplitude: P = 0.6800 |
CRMP2f/f Control males: n = 3 (11 cells) CRMP2f/f GFAP Cre males: n = 3 (16 cells) |
|
| Fig 7A | Naïve male and female CRMP2f/f/ mice – Hot Plate at 52°C | Mann-Whitney test Control males vs CaMKII Cre males: P = 0.0947 Control females vs CaMKII Cre females: P = 0.1088 |
CRMP2f/f Control males: n = 10 CRMP2f/f CaMKII Cre males: n = 9 CRMP2f/f Control females: n = 10 CRMP2f/f CaMKII Cre females: n= 10 |
|
| Fig 7B | Naïve male and female CRMP2f/f/ mice – Tail flick at 52°C | Mann-Whitney test Control males vs CaMKII Cre males: P = 0.1320 Control females vs CaMKII Cre females: P = 0.2495 |
CRMP2f/f Control males: n = 10 CRMP2f/f CaMKII Cre males: n = 8 CRMP2f/f Control females: n = 8 CRMP2f/f CaMKII Cre females: n= 10 |
|
| Fig 7D | Spared Nerve Injury in CRMP2f/f male mice – paw withdrawal threshold | Two-way ANOVA with the Sidak post hoc test | Control males vs CaMKII Cre males: Baseline P = 0.> 0.999 Post P > 0.9999 7 days P < 0.0001 14 days P < 0.0001 22 days P < 0.0001 35 days P = 0.0090 |
CRMP2f/f Control males: n = 8 CRMP2f/f CaMKII Cre males: n = 7 |
| Fig 7E | Spared Nerve Injury in CRMP2f/f male mice – Area under the curve | Mann-Whitney test Control males vs CaMKII Cre males: P = 0.0022 |
||
| Fig 7F | Spared Nerve Injury in CRMP2f/f female mice – paw withdrawal threshold | Two-way ANOVA with the Sidak post hoc test | Control females vs CaMKII Cre females: Baseline 0.9989 Post P > 0.9999 7 days P = 0.0126 14 days P = 0.0392 22 days P = 0.0523 35 days P = 0.0291 |
CRMP2f/f Control females: n = 7 CRMP2f/f CaMKII Cre females: n = 7 |
| Fig 7G | Spared Nerve Injury in CRMP2f/f female mice – Area under the curve | Mann-Whitney test Control females vs CaMKII Cre females: P = 0.0262 |
||
| Figure 8B | Spared Nerve Injury in CRMP2f/f male mice – paw withdrawal threshold | Two-way ANOVA with the Sidak post hoc test | Control males vs CaMKII Cre males: Baseline > 0.9999 7 days P = 0.0264 14 days P = 0.0499 22 days P = 0.0008 28 days P = 0.0002 36 days P < 0.0001 |
CRMP2f/f Control males: n = 10 CRMP2f/f CaMKII Cre males: n = 11 |
| Fig 8C | Spared Nerve Injury in CRMP2f/f male mice – Area under the curve | Mann-Whitney test Control males vs CaMKII Cre males: P < 0.0001 |
||
| Fig 8D | Spared Nerve Injury in CRMP2f/f female mice – paw withdrawal threshold | Two-way ANOVA with the Sidak post hoc test | Control females vs CaMKII Cre females: Baseline > 0.9999 7 days P = 0.2664 14 days P = 0.0314 22 days P < 0.0001 28 days P < 0.0001 36 days P < 0.0001 |
CRMP2f/f Control females: n = 5 CRMP2f/f CaMKII Cre females: n = 8 |
| Fig 8E | Spared Nerve Injury in CRMP2f/f female mice – Area under the curve | Mann-Whitney test Control females vs CaMKII Cre females: P = 0.0005 |
3. Results
3.1. Spinal knockdown of CRMP2 reduces the frequency and amplitude of spontaneous excitatory postsynaptic currents (sEPSCs), but not spontaneous inhibitory postsynaptic currents (sIPSCs) in the lumbar dorsal horn.
To interrogate the role of CRMP2 in spinal neurotransmission, we first used an in vivo transfection strategy to knockdown the expression of CRMP2 using a previously validated siRNA directed against CRMP2 [45]. Intrathecal injection of siRNAs against CRMP2 or a scramble sequence (control) into the spinal cord of young rats (P10–15) (Fig. 1A) resulted in a >80% reduction in CRMP2 protein levels in both the dorsal root ganglia (DRG) and lumbar dorsal horn: rats injected with CRMP2 siRNA had significantly lower levels of CRMP2 in DRG neurons (18.21 ± 2.253 (CRMP2-siRNA) vs. 100.0 ± 8.518 (control-siRNA), p<0.001) as well as in the dorsal horn (16.38 ± 7.135 (CRMP2-siRNA) vs. 100.0 ± 7.357, p<0.001) compared to scramble (control)-siRNA injected rats (Fig. 1B, C).
To determine whether the function of neurons transfected with CRMP2 siRNA are changed, we performed electrophysiological analyses in whole-cell configuration to measure spontaneous excitatory postsynaptic currents (sEPSCs) of neurons in the Substantia Gelatinosa (SG) region of the lumbar dorsal horn. Representative traces for sEPSCs are shown in Figure 1D. We found that the deletion of CRMP2 resulted in a significant decrease in both the frequency (1.6 ± 1.01 Hz (CRMP2-siRNA) vs. 2.91 ± 0.96 Hz (control-siRNA), p = 0.0005) and the amplitude (15.2 ± 4.29 pA (CRMP2-siRNA) vs. 21.05 ± 5.62 pA (control-siRNA), p = 0.0033) of sEPSCs (Fig. 1E, F).
Decreased inhibitory transmission is an important component of chronic pain states [10; 14]. However, while CRMP2 targeting decreases excitatory transmission (Fig. 1) and provides pain relief [37–40; 45–49], it is not known if CRMP2 can act to enhance spinal inhibitory tone. Therefore, we next isolated inhibitory synapses by pharmacologically blocking α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)- and N-methyl-D-aspartate (NMDA)-activated currents (see Methods) and measured sIPSCs in neurons in the SG region of the lumbar dorsal horn. Representative traces for sIPSCs are shown in Figure 2A. We found that both the frequency and the amplitude of sIPSCs were not different in slices prepared from rats injected with CRMP2- or scramble-siRNA (Fig. 2B, C), thus showing that CRMP2 has no role to play in spinal inhibitory neurotransmission.
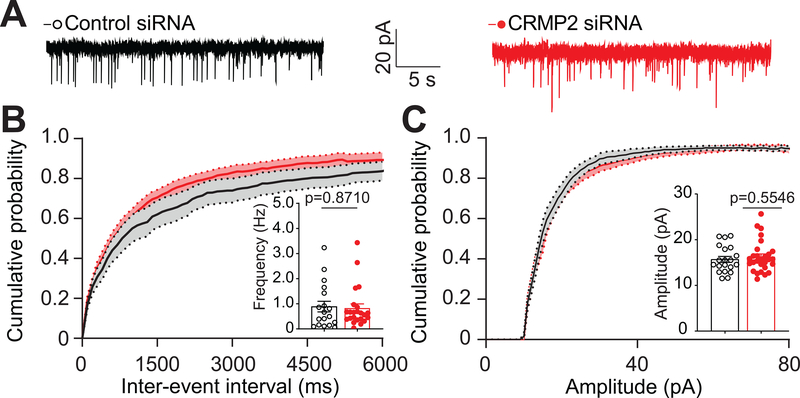
Fig 2. siRNA-mediated knockdown of CRMP2 does not affect the frequency or amplitude of spontaneous inhibitory postsynaptic currents (sIPSCs) in the Substantia gelatinosa region of the lumbar dorsal horn.
A. Representative traces of sIPSC recordings from SG neurons from the indicated groups. B. Cumulative distribution of sIPSC inter-event intervals and bar graph with scatter plot summary of sIPSC frequencies. No significant change was observed. C. Cumulative distribution and bar graph with scatter plot summary of sIPSC amplitudes. No significant change was observed. Data are expressed as means ± SEM. Non-parametric Mann-Whitney test. For full statistical analyses, see Table 1. sIPSC: spontaneous Inhibitory Postsynaptic Currents, SG: Substantia Gelatinosa.
Together, these results indicate that CRMP2 is involved exclusively in excitatory glutamatergic neurotransmission but not at inhibitory glycinergic and GABAergic synapses in the spinal dorsal horn. CRMP2 functions may be restricted to glutamatergic nociceptive synapses in the lamina I/II [21; 54].
3.2. Spinal knockdown of CRMP2 has no effect on either miniature excitatory or inhibitory postsynaptic currents in the lumbar dorsal horn.
CRMP2 can regulate the release of the excitatory neurotransmitter glutamate and CGRP [3; 8; 38; 45]. To dissociate neurotransmitter release from synaptic activity, we added tetrodotoxin (TTX, 1μM), a blocker of voltage-gated sodium channels (VGSCs), to the recording solution (see Methods). By blocking VGSCs, TTX prevents membrane depolarization, which consequently prevents the activation of voltage-gated calcium channels (VGCCs) and the subsequent ingress of calcium in neurons. Under these recording conditions, the neurotransmitter release observed is solely due to residual calcium activity only and occurs as a slow vesicle exocytosis [52; 55]. This allows us to record, in the whole-cell configuration, miniature excitatory and inhibitory postsynaptic currents (mEPSCs and mIPSCs, respectively) of neurons in the SG region of lumbar dorsal horn of rats transfected with either a control siRNA or CRMP2 siRNA. Representative traces for mEPSCs and mIPSCs are shown in Figure 3. No change was observed in the frequency and amplitude of mEPSCs (Fig. 3A–C) and mIPSCs (Fig. 3D–F) between slices prepared from rats injected with control and CRMP2 siRNAs. These observations confirm that CRMP2 function in neurotransmission is dependent on presynaptic activity.
Fig 3. siRNA-mediated knockdown of CRMP2 does not affect miniature excitatory or inhibitory postsynaptic currents in the lumbar dorsal horn.
A. Representative traces of mEPSC recordings from SG neurons transfected either with a control siRNA or an siRNA targeting CRMP2 (CRMP2 siRNA). Cumulative distribution of mEPSC inter-event intervals B, and amplitudes C. Insets are bar graph with scatter plot summaries of mEPSC frequencies and amplitudes as indicated. No significant change was observed. D. Representative traces of mIPSC recordings from SG neurons from the indicated groups. Cumulative distribution of mIPSC inter-event intervals E and amplitudes F. Insets are bar graph with scatter plot summaries of mIPSC frequencies and amplitudes. No significant change was observed between the conditions. Data are expressed as ± SEM. Unpaired t-test with Welch’s correction. See Table 1. for full statistical analyses. mEPSC: miniature Excitatory Postsynaptic Currents, mIPSC: miniature Inhibitory Postsynaptic Currents, SG: Substantia Gelatinosa.
3.3. Conditional knockout of CRMP2 in mouse glutamatergic neurons reduces the frequency and amplitude of spontaneous, but not miniature, excitatory postsynaptic currents in the lumbar dorsal horn.
Using an siRNA specifically silencing CRMP2, we found that CRMP2 contribution to nociceptive spinal neurotransmission may be located exclusively at glutamatergic synapses. This approach allowed us to rule out an involvement of CRMP2 in inhibitory neurotransmission (Figure 2) and in activity independent excitatory or inhibitory neurotransmitter release (Figure 3). To investigate the cell-specific contribution of CRMP2, we used a transgenic mouse line where LoxP sequences flank the exon 3 of the dpysl2 gene (designated as CRMP2f/f in this manuscript)[43]. Mice were injected intrathecally with an adeno-associated virus (AAV9) expressing the recombinase Cre under the control of the calcium/calmodulin dependent protein kinase II alpha (CaMKIIα) promoter; CaMKIIα is a marker of glutamatergic neurons in the central nervous system [16; 25; 31] and is expressed in spinal dorsal horn’s superficial laminae and DRG neurons involved in nociceptive pathways [11]. Using this CaMKIIα-GFP-CRE AAV9; we tested if conditional knockout of CRMP2 from glutamatergic neurons could recapitulate our observations in rats injected with a CRMP2 siRNA (Figure 1). Seven days following the intrathecal injections of either the CaMKIIα-GFP-CRE AAV9 or the control AAV (CaMKIIα-eGFP), we observed an ~50% reduction in the amount of CRMP2 protein in the DRG and spinal cords harvested from CaMKIIα-GFP-CRE AAV9 infected CRMP2f/f mice: CaMKIIα-GFP-CRE- AAV9 54.09 ± 6.35 vs. CaMKIIα-eGFP-AAV9 100.00 ± 13.89 (DRG), p = 0.0065); CaMKIIα-GFP-CRE- AAV9 45.15 ± 2.90 vs. CaMKIIα-eGFP-AAV9 100.00 ± 8.75, p = 0.0007 (dorsal horn) (Figure 4).
Fig 4. Validation of conditional knockout of CRMP2 in mouse dorsal root ganglia (DRG) and spinal dorsal horn (SDH).
A. Representative immunoblots showing the expression of CRMP2 in the DRG and SDH of mice with either a control AAV (CaMKIIα-GFP) or an AAV deleting CRMP2 in CaMKIIα+ neurons (CaMKIIα-CRE). Samples were harvested 7 days following injections. βIII-Tubulin is used as a loading control. B. Bar graph with scatter plot showing decreased CRMP2 expression in DRG and SDH of mice injected with the AAV allowing for expression of the recombinase Cre under the control of the CaMKII promoter (CaMKII-CRE) compared to control. Data are expressed as mean ± SEM. Unpaired t-test with Welch’s correction. For full statistical analyses, see Table 1. DRG: Dorsal Root Ganglion, SDH: Spinal Dorsal Horn.
Next, to determine whether glutamatergic neuron-specific conditional knockout of CRMP2 changes synaptic transmission in the dorsal spinal cord, we recorded sEPSCs in the SG region of lumbar dorsal horn of young CRMP2f/f mice (P10–15) (Figure 5A). We found that the conditional knockout of CRMP2 in CaMKIIα+ neurons (identified by GFP fluorescence) of the SDH resulted in a significant decrease in both the frequency (CaMKIIα-GFP-CRE- AAV9 0.978 ± 0.51 vs. CaMKIIα-eGFP-AAV9 2.81 ± 1.59 Hz, p = 0.0014) and the amplitude (CaMKIIα-GFP-CRE-AAV9 15.33 ± 1.79 vs. CaMKIIα-eGFP-AAV9 23.73 ± 7.68 pA, p = 0.0020) of sEPSCs compared to control mice (CaMKIIα-eGFP) (Figures 5B, C). These results are entirely congruent with data obtained from rat spinal cord slices transfected with the CRMP2 siRNA (Figure 1), supporting a role for CRMP2 in regulating neurotransmission at glutamatergic synapses (i.e. CaMKIIα+ neurons).
Fig 5. Conditional knockout of CRMP2 in mouse neurons reduces the frequency and amplitude of spontaneous excitatory postsynaptic currents (sEPSCs) but does not affect miniature excitatory postsynaptic currents (mEPSCs) in the lumbar dorsal horn.
A. Representative traces of sEPSC recordings from SG neurons transduced with a control AAV (CaMKIIα-GFP) or an AAV allowing for expression of the recombinase Cre under the control of the CaMKII promoter (CaMKIIα-CRE). B. Cumulative distribution of sEPSC inter-event intervals, and summary of sEPSC frequencies recorded from SG neurons from mice injected with CaMKIIα-CRE AAV revealed a right shift towards longer inter-event intervals compared to control, as well as a significant decrease in the frequency (inset) compared to control. C. Cumulative distribution and summary of sEPSC amplitudes from mice transfected with CaMKII-CRE revealed a left shift toward smaller amplitudes compared to control, as well as a significant decrease in amplitude (inset) compared to control. D. Representative traces of mEPSC recordings from SG neurons transduced with the indicated AAV. Cumulative distributions of mEPSC inter-event intervals E, and amplitudes F., and bar graph with scatter plot summaries of mEPSC frequencies and amplitudes. No significant change was observed. Data are expressed as ± SEM. Unpaired t-test with Welch’s correction. For full statistical analyses, see Table 1. sEPSC: spontaneous Excitatory Postsynaptic Currents, mEPSC: miniature Excitatory Postsynaptic Currents, SG: Substantia Gelatinosa, CaMKIIα: Calcium/Calmodulin-dependent protein Kinase IIα.
Next, we interrogated whether conditional knockout of CRMP2 in CaMKIIα+ neurons would affect neurotransmitter release in the absence of synaptic activity. As before (Figure 3), we added TTX (1μM) and measured mEPSCs from neurons of the SG region of the lumbar dorsal horn of the CRMP2f/f mice (Figure 5D). Under conditions when presynaptic activity is blocked, we observed no effect on the frequency and the amplitude of mEPSCs in CaMKIIα-GFP-CRE-AAV9- versus CaMKIIα-eGFP-AAV9-infected slices (Figures 5E, F). These results are consistent with the results obtained from rat spinal cord slices treated with CRMP2 siRNA. Together our data replicated in two different species (rats and mice) and using two different genetic tools (siRNA- and AAV-driven selective conditional knockout of CRMP2), demonstrate that the role of CRMP2 in spinal neurotransmission is restricted to the presynaptic regulation of excitatory (CaMKIIα+) synapses.
3.4. CRMP2 expressed in astrocytes does not play a role in spinal transmission.
Astrocytes are widely present in the central nervous system where they contribute to homeostasis and play an important role in spinal neurotransmission [24]. Additionally, astrocytes have been linked to neuropathic pain [27; 50]. Low levels of CRMP2 messenger RNA have been detected in adult mouse astrocytes [62]. However, this observation was not replicated by another study [60]. Our attempts at detecting CRMP2 expression in spinal astrocytes using immunohistofluorescence did not yield any trustworthy signal [42]. To impartially address a potential role of CRMP2 in spinal astrocytes, we injected CRMP2f/f mice [43] with an adeno-associated virus serotype 5 (AAV5) expressing the recombinase Cre under the control of the glial fibrillary acid protein (GFAP) promoter; GFAP is a marker of astrocytes [5; 30]. We could not control for the loss of CRMP2 protein expression in these conditions as the presence of the protein itself remains controversial [42; 48; 60; 62]. Two days following the intrathecal injections of either the GFAP-CRE AAV5 or the GFAP-eGFP AAV5 (control), we recorded sEPSCs in the SG region of lumbar dorsal horn of young CRMP2f/f mice (P10–15) (Figure 6A). The conditional knockout of CRMP2 in spinal GFAP+ astrocytes had no effect on the frequency and the amplitude of sEPSCs of spinal neurons compared to neurons prepared from GFAP-eGFP AAV5 infected CRMP2f/f mice (Figures 6B, C). Because our earlier experiments in rats did not highlight a role of CRMP2 in inhibitory transmission (Figure 3), we did not record sIPSCs from lumbar dorsal horn in neurons from GFAP-CRE AAV5-transduced mice. These observations support the conclusion that CRMP2 expressed in astrocytes does not play a role in spinal neurotransmission.
Fig 6. CRMP2 expressed in astrocytes does not play a role in spinal transmission.
A. Representative traces of sEPSC recordings from SG neurons from either control (GFAP-GFP) or CRMP2 deleted (GFAP-CRE) mice. B. Cumulative distribution of sEPSC inter-event intervals and bar graph with scatter plot summary of sEPSC frequencies (inset). Deleting CRMP2 in spinal astrocytes did not affect these parameters. C. Cumulative distribution and bar graph with scatter plot summary of sEPSC amplitudes (inset). Deletion of CRMP2 in spinal astrocytes had no effect. Data are expressed as means ± SEM. Nonparametric Mann-Whitney test. For full statistical analyses, see Table 1. sEPSC: spontaneous Excitatory Postsynaptic Currents, SG; Substantia Gelatinosa, GFAP: Glial Fibrillary Acidic Protein.
3.5. Conditional knockout of CRMP2 in mice reverses mechanical allodynia in the spared nerve injury (SNI) model of chronic neuropathic pain without affecting physiological nociception.
With the above series of experiments, we have shown the role of CRMP2 in maintaining neurotransmission at glutamatergic synapses in the spinal dorsal horn of rats and mice. Spinal neurotransmission in lamina I and II of the spinal dorsal horn relies on stimuli conveyed by the nociceptive fibers (C-fibers and Aδ-nociceptors [21; 54]). As conditional knockout of CRMP2 in glutamatergic neurons in the spinal cord of CRMP2f/f mice significantly disrupts synaptic transmission, we expect that it will also affect nociception, resulting in behavioral changes. We used adult naïve CRMP2f/f mice injected intrathecally with either CaMKIIα-eGFP (control) or CaMKIIα-GFP-CRE AAV9 and measured their responses to acute noxious thermal stimuli. The hot plate assay measures supraspinal responses to nociception, and tail-flick assay, which interrogates reflexive nociception responses tests – both were conducted at 52°C in naïve male and female CRMP2f/f mice. We found no difference in the withdrawal latencies between naïve mice, of either sex, injected with CaMKIIα-GFP-CRE AAV9 or CaMKIIα-eGFP AAV9 (control) (Figure 7A). Additionally, we found no difference in the tail-flick latencies between both groups in either sex (Figure 7B). Together, these results indicate that conditional knockout of neuronal CRMP2 in the spinal cord does not impair the ability of these mice to process acute thermal nociception.
Fig 7. Conditional knockout of neuronal CRMP2 in mice reverses mechanical allodynia in the spared nerve injury (SNI) model of chronic neuropathic pain without affecting physiological nociception.
A. Paw withdrawal and B. Tail-flick latency at 52°C in naïve male and female mice injected with either CaMKII-GFP (Control) or CaMKII-CRE (CRMP2 knockout in CaMKII+ neurons). No significant change was observed in the response latency between the indicated groups for both male and female mice. Nonparametric Mann-Whitney test. CRMP2f/f mice with a spared nerve injury (SNI) were injected with either CaMKII-GFP or CaMKII-CRE AAV over 35 days. Timeline (C), time course (D – males; F – females) and area under the curve (E – males; G – females) are shown. SNI elicited mechanical allodynia 15 days after surgery. Conditional knockout of CRMP2 in CaMKII+ neurons reversed mechanical allodynia compared to control. Data are shown as mean ± SEM and were analyzed by nonparametric two-way analysis of variance where time was the within-subject factor and treatment was the between-subject factor (post hoc: Sidak) (D, F) and by nonparametric Mann-Whitney test (E, G). The experiments were analyzed by an investigator blinded to the treatment. P values of comparisons between treatments are as indicated; for full statistical analyses, see Table 1. Blue arrows: AAV injection, SNI: Spared Nerve Injury.
To test the contribution of CRMP2 expressed in CaMKIIα+ neurons to chronic neuropathic pain, we performed SNI surgery in male and female mice CRMP2f/f and 15 days following surgery (post-SNI), we injected the mice with CaMKIIα-GFP-CRE-AAV9 or CaMKIIα-eGFP-AAV9 (control) (Figure 7C). Mechanical allodynia was followed once weekly post injection for 4 weeks. Fifteen days post-surgery male and female mice developed pronounced mechanical allodynia (Figures 7 D–G) which sustained over the course of the four-week testing period in the CaMKIIα-eGFP-AAV9 injected control groups (Figures 7D, F). CRMP2f/f mice injected with CaMKIIα-CRE AAV9 showed a prolonged reversal of chronic mechanical allodynia irrespective of sex: males – CaMKIIα-GFP-CRE 8.62 ± 0.00 g vs. CaMKIIα-eGFP 1.08 ± 0.00 g, p = 0.0022) (Figure 7E), females –CaMKIIα-GFP-CRE 7.26 ± 0.00 g vs. CaMKIIα-eGFP 1.13 ± 0.00 h, p = 0.0262) (Figure 7G). At the four week point following SNI, the paw withdrawal threshold was almost indistinguishable from its pre-surgery level. Together, these results show that deleting CRMP2 in the spinal cord can permanently reverse chronic mechanical allodynia in both male and female mice with chronic neuropathic pain. This suggests that CRMP2 may be critical for the maintenance of chronic pain.
We next asked if CRMP2 was similarly important for the development of chronic pain. We injected CRMP2f/f mice with either CaMKIIα-GFP-CRE-AAV9 or CaMKIIα-eGFP-AAV9 and then subjected them to a SNI surgery (Figure 8A). Before surgery, CaMKIIα-GFP-CRE-AAV9 or CaMKIIα-eGFP-AAV9 injected male (Figure 8B, C) and female groups were indistinguishable (Figure 8D, E), demonstrating that CRMP2 deletion does not impact basal mechanical allodynia in naïve mice (Fig. 8B, D; day 0). Seven days following SNI, mice (both sexes) injected with CaMKIIα-GFP-CRE AAV9 had higher paw withdrawal thresholds than those injected with control (CaMKIIα-GFP-CRE) virus (Figure 8B, D). CaMKIIα-eGFP-AAV9 injected mice (both sexes) developed sustained mechanical allodynia while the paw withdrawal threshold of mice injected with CaMKIIα-GFP-CRE-AAV9 gradually reversed to their pre-surgery levels (p<0.0001) (Figure 8B, D). Cumulative data inferred from area under the curve corroborated near complete prevention of the development of mechanical allodynia in mice of both sexes (Figure 8C, E). Together, these observations demonstrate that deleting CRMP2 can not only reverse but also can even prevent the development of chronic neuropathic pain (neuropathic pain being considered as established at 14 days following SNI, [9; 12]).
Fig 8. Conditional knockout of CRMP2 in CaMKIIα+ neurons prevents mechanical allodynia in mice with a spared nerve injury (SNI).
CRMP2f/f mice were injected with either CaMKIIα-eGFP or CaMKIIα-GFP-Cre AAV before spared nerve injury (SNI). Timeline (A), time course (B – males; D females) and area under the curve (C – males; E – females) are shown. SNI elicited mechanical allodynia 7 days after surgery in control mice. Conditional knockout of CRMP2 in CaMKII+ neurons prevented mechanical allodynia in male mice (p=0.00264), and reversed mechanical allodynia compared to control in both male and female CRMP2f/f mice. Data are shown as mean ± SEM and were analyzed by non-parametric two-way analysis of variance where time was the within-subject factor and treatment was the between-subject factor (post hoc: Sidak) (B, D) and by non-parametric Mann-Whitney test (C, E). The experiments were analyzed by an investigator blinded to the treatment. P values of comparison between treatments are as indicated, for full statistical analyses see Table 1. Blue arrows: AAV injection, SNI: Spared Nerve Injury
4. Discussion
In this study, our goal was to elucidate the fundamental properties of CRMP2 in spinal nociceptive pathways using two different species (rat and mice) and two different genetic approaches (siRNA and Cre/loxP). We first knocked-down the expression of CRMP2 in the dorsal root ganglion (DRG) and in the spinal dorsal horn (SDH) of young rats (P10–15) with a selective siRNA. We found an exclusive presynaptic function of CRMP2 in excitatory but not inhibitory spinal neurotransmission. In transgenic mice, deleting CRMP2 in excitatory neurons replicated our findings in rats. We also showed that CRMP2 expression in glial cells had no function in spinal neurotransmission. We found that deleting CRMP2 from excitatory neurons could reverse or prevent chronic mechanical allodynia in a mouse model of chronic neuropathic pain (SNI). CRMP2 had no effect on acute nociceptive pain. Our data strongly demonstrate that CRMP2 is a key regulator of glutamatergic neurotransmission driving pain signaling and contributes to the transition from physiological to pathological pain.
The present studies allowed us to narrow the role of CRMP2 to glutamatergic transmission in the spinal cord. This is supported by several salient findings. First, suppression of CRMP2 expression decreased sEPSC frequency and amplitude in rats and mice. Second, there was no effect on inhibitory postsynaptic currents (sIPSCs) recorded from superficial dorsal horn neurons in acute spinal cord slices. Third, no effect was noted on miniature excitatory (mEPSCs) and inhibitory postsynaptic currents (mIPSCs). Single cell RNA-seq studies found negligible traces of CRMP2 (dpysl2) transcripts in GABAergic neurons in the spinal cord [22; 60], thus supporting our findings of CRMP2’s action being restricted to glutamatergic synapses in the SDH. Miniature currents are recorded in presence of TTX, a blocker of VGSCs, which allows for the separation of neurotransmitter release from presynaptic activity. A post-synaptic action of CRMP2 would have been supported by a diminution of the amplitude of the miniature currents. Nevertheless, the impact of loss of CRMP2 on sEPSC amplitude is significant. This may be explained by an atypical function of CRMP2 as a neurotransmitter [44]. CRMP2 can be released from injured nerves and directly activate N-methyl-D-aspartate (NMDA) receptors [4]. These receptors are localized both pre- and post-synaptically in the spinal dorsal horn [13; 63]. Loss of their activation via extracellular CRMP2 may account for at least part of the decreased frequency and amplitude of sEPSCs observed in our studies. Thus, a reasonable inference from our findings is that CRMP2 function is limited to the pre-synaptic sites of the SDH.
Our goal was to perform a comprehensive analysis of the role of CRMP2 in spinal neurotransmission. In this regard, we included astrocytes because of their important modulatory and pathological role in chronic pain [27; 50]. Whether CRMP2 is expressed in these cells remains controversial with reports for [62] and against [42; 60] the presence of transcripts. We used the CRMP2f/f mice to directly test if the dpysl2, the gene coding for CRMP2, could have a function in modulating spinal excitatory transmission. Our results unequivocally show that CRMP2 expression (if any) in astrocytes has no impact on nociceptive signal propagation at the spinal level. Microglia were not tested because of the lack of evidence of CRMP2 expression in these cells [42; 60].
Deleting CRMP2 in CaMKIIα+ (excitatory) neurons had no effect in the hot-plate and the tail-flick tests, suggesting that CRMP2 does not impact physiological (i.e., protective) pain responses. At the spinal level, residual neurotransmission following CRMP2 deletion could be sufficient to permit acute nociceptive pain signals. These findings are particularly encouraging as a ‘de-risking’ step for future therapeutic targeting of CRMP2. Equally exciting are our salient findings that suppression of CRMP2 can both prevent the development of and reverse established chronic mechanical allodynia in mice with SNI. This observation is supported by the observation that mice with loss of CRMP2 in glutamatergic neurons had detectable allodynia one week after SNI, although it was lower than controls. This key result shows that inhibiting CRMP2 can not only mitigate allodynia but can also block the chronification of neuropathic pain. Congruent with this observation is our previous finding that conditional knockout of CRMP2 from excitatory neurons (CaMKIIα+) prevented gliosis in a model of experimental autoimmune encephalomyelitis [43]. Glial activation in the spinal cord is a marker for chronic neuropathic pain [34]. Increased excitatory synaptic activity in the spinal cord triggers astrogliosis [34; 64]. In turn, activated astrocytes produce brain derived neurotrophic factor (BDNF) to sensitize sensory neurons [19; 64], thereby activating a positive feedback loop via its tyrosine receptor kinase B (TrkB), a neurotrophin receptor, to produce long term potentiation (LTP) which is hypothesized to underlie chronic pain [64]. CRMP2 is part of a protein complex with Slp1 (also known as JFC1 or Exophilin-7) and the GTPase Rab27B, linking TrkB to the anterograde motor kinesin 1 [1]. Loss of CRMP2 reduced membrane targeting of TrkB, consequently preventing activation of BDNF signaling in neurons [1]. We propose that deleting CRMP2 (CRMP2f/f mice) prior to a spared nerve injury prevented the establishment of chronic neuropathic pain via (i) silenced spinal neurotransmission which (ii) blocked spinal astrogliosis and (iii) subsequent BDNF production. Together, these actions may put a ‘brake’ to the positive feedback loop leading to spinal LTP. In this context, activated microglia may be alternative sources of BDNF in the spinal cord [64]. However, since CRMP2 can also control the membrane targeting of the BDNF receptor TrkB and downstream signaling [1], conditional knockout of CRMP2 would ultimately prevent any BDNF-mediated potentiation at the primary afferents. This suggests that targeting CRMP2 can not only curb excitatory spinal transmission but also negate any potential sensitization by BDNF. Thus, inhibiting CRMP2 for the treatment of chronic pain may be disease-modifying and reverse persistent pain permanently. With the rising interest in using gene therapy for treating high impact chronic pain [35; 36], our data shows that dpysl2 (coding for CRMP2) is an additional gene of interest for these endeavors. This is further supported by our previous report that knockdown of CRMP2 reversed mechanical allodynia in a rat model of SNI [45].
Our goal was to perform a comprehensive analysis of the role of CRMP2 in spinal neurotransmission. The spinal cord is the first site for the integration of a nociceptive signal, its modulation holds substantial therapeutic potential. Our results confirm previous reports and show that CRMP2 participates both in the maintenance as well as the initiation of chronic neuropathic pain following a nerve injury. Also important is our finding that physiological pain is independent of CRMP2 expression. This indicates that future therapies targeting this protein are less likely to be associated with the common side effects, including numbness seen in clinically used painkillers. Together, our data suggests that targeting CRMP2 is a safe way to prevent and reverse chronic neuropathic pain.
Acknowledgments
Funding
This work is supported by National Institutes of Health awards from NINDS (NS098772 and NS120663 to R.K. and NS119263 to A.M.), NIDA (DA042852 to R.K.) and a grant from the National Natural Science Foundation of China (81971052 to J.Y.).
Conflict of interest statement
R. Khanna is the cofounder of Regulonix LLC, a company developing nonopioid drugs for chronic pain. In addition, R. Khanna has patents US10287334 and US10441586 issued to Regulonix LLC. The remaining authors have no conflicts of interest to declare.
References
- [1].Arimura N, Kimura T, Nakamuta S, Taya S, Funahashi Y, Hattori A, Shimada A, Menager C, Kawabata S, Fujii K, Iwamatsu A, Segal RA, Fukuda M, Kaibuchi K. Anterograde transport of TrkB in axons is mediated by direct interaction with Slp1 and Rab27. Dev Cell 2009;16(5):675–686. [DOI] [PubMed] [Google Scholar]
- [2].Brittain JM, Chen L, Wilson SM, Brustovetsky T, Gao X, Ashpole NM, Molosh AI, You H, Hudmon A, Shekhar A, White FA, Zamponi GW, Brustovetsky N, Chen J, Khanna R. Neuroprotection against traumatic brain injury by a peptide derived from the collapsin response mediator protein 2 (CRMP2). The Journal of biological chemistry 2011;286(43):37778–37792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Brittain JM, Piekarz AD, Wang Y, Kondo T, Cummins TR, Khanna R. An atypical role for collapsin response mediator protein 2 (CRMP-2) in neurotransmitter release via interaction with presynaptic voltage-gated calcium channels. The Journal of biological chemistry 2009;284(45):31375–31390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Castillo C, Martinez JC, Longart M, Garcia L, Hernandez M, Carballo J, Rojas H, Matteo L, Casique L, Escalona JL, Rodriguez Y, Rodriguez J, Hernandez D, Balbi D, Villegas R. Extracellular Application of CRMP2 Increases Cytoplasmic Calcium through NMDA Receptors. Neuroscience 2018;376:204–223. [DOI] [PubMed] [Google Scholar]
- [5].Chan KY, Jang MJ, Yoo BB, Greenbaum A, Ravi N, Wu WL, Sanchez-Guardado L, Lois C, Mazmanian SK, Deverman BE, Gradinaru V. Engineered AAVs for efficient noninvasive gene delivery to the central and peripheral nervous systems. Nat Neurosci 2017;20(8):1172–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Chew LA, Bellampalli SS, Dustrude ET, Khanna R. Mining the Nav1.7 interactome: Opportunities for chronic pain therapeutics. Biochemical pharmacology 2019;163:9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chew LA, Khanna R. CRMP2 and voltage-gated ion channels: potential roles in neuropathic pain. Neuronal Signal 2018;2(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chi XX, Schmutzler BS, Brittain JM, Wang Y, Hingtgen CM, Nicol GD, Khanna R. Regulation of N-type voltage-gated calcium channels (Cav2.2) and transmitter release by collapsin response mediator protein-2 (CRMP-2) in sensory neurons. J Cell Sci 2009;122(Pt 23):4351–4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cichon J, Sun L, Yang G. Spared Nerve Injury Model of Neuropathic Pain in Mice. Bio Protoc 2018;8(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].D’Mello R, Dickenson AH. Spinal cord mechanisms of pain. Br J Anaesth 2008;101(1):8–16. [DOI] [PubMed] [Google Scholar]
- [11].Dai Y, Wang H, Ogawa A, Yamanaka H, Obata K, Tokunaga A, Noguchi K. Ca2+/calmodulin-dependent protein kinase II in the spinal cord contributes to neuropathic pain in a rat model of mononeuropathy. Eur J Neurosci 2005;21(9):2467–2474. [DOI] [PubMed] [Google Scholar]
- [12].Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain 2000;87(2):149–158. [DOI] [PubMed] [Google Scholar]
- [13].Deng M, Chen SR, Pan HL. Presynaptic NMDA receptors control nociceptive transmission at the spinal cord level in neuropathic pain. Cell Mol Life Sci 2019;76(10):1889–1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Dickenson AH. Spinal cord pharmacology of pain. Br J Anaesth 1995;75(2):193–200. [DOI] [PubMed] [Google Scholar]
- [15].Dustrude ET, Moutal A, Yang X, Wang Y, Khanna M, Khanna R. Hierarchical CRMP2 posttranslational modifications control NaV1.7 function. Proceedings of the National Academy of Sciences of the United States of America 2016;113(52):E8443–E8452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Fink CC, Meyer T. Molecular mechanisms of CaMKII activation in neuronal plasticity. Curr Opin Neurobiol 2002;12(3):293–299. [DOI] [PubMed] [Google Scholar]
- [17].Francois-Moutal L, Dustrude ET, Wang Y, Brustovetsky T, Dorame A, Ju W, Moutal A, Perez-Miller S, Brustovetsky N, Gokhale V, Khanna M, Khanna R. Inhibition of the Ubc9 E2 SUMO-conjugating enzyme-CRMP2 interaction decreases NaV1.7 currents and reverses experimental neuropathic pain. Pain 2018;159(10):2115–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Fukata Y, Itoh TJ, Kimura T, Menager C, Nishimura T, Shiromizu T, Watanabe H, Inagaki N, Iwamatsu A, Hotani H, Kaibuchi K. CRMP-2 binds to tubulin heterodimers to promote microtubule assembly. Nature cell biology 2002;4(8):583–591. [DOI] [PubMed] [Google Scholar]
- [19].Gao YJ, Ji RR. Targeting astrocyte signaling for chronic pain. Neurotherapeutics 2010;7(4):482–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Goshima Y, Nakamura F, Strittmatter P, Strittmatter SM. Collapsin-induced growth cone collapse mediated by an intracellular protein related to UNC-33. Nature 1995;376(6540):509–514. [DOI] [PubMed] [Google Scholar]
- [21].Harding EK, Fung SW, Bonin RP. Insights Into Spinal Dorsal Horn Circuit Function and Dysfunction Using Optical Approaches. Front Neural Circuits 2020;14:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Haring M, Zeisel A, Hochgerner H, Rinwa P, Jakobsson JET, Lonnerberg P, La Manno G, Sharma N, Borgius L, Kiehn O, Lagerstrom MC, Linnarsson S, Ernfors P. Neuronal atlas of the dorsal horn defines its architecture and links sensory input to transcriptional cell types. Nat Neurosci 2018;21(6):869–880. [DOI] [PubMed] [Google Scholar]
- [23].Inagaki N, Chihara K, Arimura N, Menager C, Kawano Y, Matsuo N, Nishimura T, Amano M, Kaibuchi K. CRMP-2 induces axons in cultured hippocampal neurons. Nature neuroscience 2001;4(8):781–782. [DOI] [PubMed] [Google Scholar]
- [24].Ji RR, Donnelly CR, Nedergaard M. Astrocytes in chronic pain and itch. Nat Rev Neurosci 2019;20(11):667–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Jones EG, Huntley GW, Benson DL. Alpha calcium/calmodulin-dependent protein kinase II selectively expressed in a subpopulation of excitatory neurons in monkey sensory-motor cortex: comparison with GAD-67 expression. J Neurosci 1994;14(2):611–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Khanna R, Zougman A, Stanley EF. A proteomic screen for presynaptic terminal N-type calcium channel (CaV2.2) binding partners. Journal of biochemistry and molecular biology 2007;40(3):302–314. [DOI] [PubMed] [Google Scholar]
- [27].Kohro Y, Matsuda T, Yoshihara K, Kohno K, Koga K, Katsuragi R, Oka T, Tashima R, Muneta S, Yamane T, Okada S, Momokino K, Furusho A, Hamase K, Oti T, Sakamoto H, Hayashida K, Kobayashi R, Horii T, Hatada I, Tozaki-Saitoh H, Mikoshiba K, Taylor V, Inoue K, Tsuda M. Spinal astrocytes in superficial laminae gate brainstem descending control of mechanosensory hypersensitivity. Nat Neurosci 2020;23(11):1376–1387. [DOI] [PubMed] [Google Scholar]
- [28].Kopach O, Voitenko N. Spinal AMPA receptors: Amenable players in central sensitization for chronic pain therapy? Channels (Austin) 2021;15(1):284–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. The journal of pain : official journal of the American Pain Society 2009;10(9):895–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lee Y, Messing A, Su M, Brenner M. GFAP promoter elements required for region-specific and astrocyte-specific expression. Glia 2008;56(5):481–493. [DOI] [PubMed] [Google Scholar]
- [31].Liu XB, Murray KD. Neuronal excitability and calcium/calmodulin-dependent protein kinase type II: location, location, location. Epilepsia 2012;53 Suppl 1:45–52. [DOI] [PubMed] [Google Scholar]
- [32].Malgaroli A, Tsien RW. Glutamate-induced long-term potentiation of the frequency of miniature synaptic currents in cultured hippocampal neurons. Nature 1992;357(6374):134–139. [DOI] [PubMed] [Google Scholar]
- [33].Manabe T, Renner P, Nicoll RA. Postsynaptic contribution to long-term potentiation revealed by the analysis of miniature synaptic currents. Nature 1992;355(6355):50–55. [DOI] [PubMed] [Google Scholar]
- [34].Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nature reviews Neuroscience 2009;10(1):23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Mohan A, Fitzsimmons B, Zhao HT, Jiang Y, Mazur C, Swayze EE, Kordasiewicz HB. Antisense oligonucleotides selectively suppress target RNA in nociceptive neurons of the pain system and can ameliorate mechanical pain. Pain 2018;159(1):139–149. [DOI] [PubMed] [Google Scholar]
- [36].Moreno AM, Aleman F, Catroli GF, Hunt M, Hu M, Dailamy A, Pla A, Woller SA, Palmer N, Parekh U, McDonald D, Roberts AJ, Goodwill V, Dryden I, Hevner RF, Delay L, Goncalves Dos Santos G, Yaksh TL, Mali P. Long-lasting analgesia via targeted in situ repression of NaV1.7 in mice. Sci Transl Med 2021;13(584). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Moutal A, Cai S, Luo S, Voisin R, Khanna R. CRMP2 is necessary for Neurofibromatosis type 1 related pain. Channels (Austin) 2018;12(1):47–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Moutal A, Cai S, Yu J, Stratton HJ, Chefdeville A, Gomez K, Ran D, Madura CL, Boinon L, Soto M, Zhou Y, Shan Z, Chew LA, Rodgers KE, Khanna R. Studies on CRMP2 SUMOylation-deficient transgenic mice identify sex-specific Nav1.7 regulation in the pathogenesis of chronic neuropathic pain. Pain 2020;161(11):2629–2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Moutal A, Chew LA, Yang X, Wang Y, Yeon SK, Telemi E, Meroueh S, Park KD, Shrinivasan R, Gilbraith KB, Qu C, Xie JY, Patwardhan A, Vanderah TW, Khanna M, Porreca F, Khanna R. (S)-lacosamide inhibition of CRMP2 phosphorylation reduces postoperative and neuropathic pain behaviors through distinct classes of sensory neurons identified by constellation pharmacology. Pain 2016;157(7):1448–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Moutal A, Dustrude ET, Largent-Milnes TM, Vanderah TW, Khanna M, Khanna R. Blocking CRMP2 SUMOylation reverses neuropathic pain. Molecular psychiatry 2018;23(11):2119–2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Moutal A, Francois-Moutal L, Brittain JM, Khanna M, Khanna R. Differential neuroprotective potential of CRMP2 peptide aptamers conjugated to cationic, hydrophobic, and amphipathic cell penetrating peptides. Front Cell Neurosci 2014;8:471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Moutal A, Ji Y, Bellampalli SS, Khanna R. Differential expression of Cdk5-phosphorylated CRMP2 following a spared nerve injury. Molecular brain 2020;13(1):97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Moutal A, Kalinin S, Kowal K, Marangoni N, Dupree J, Lin SX, Lis K, Lisi L, Hensley K, Khanna R, Feinstein DL. Neuronal Conditional Knockout of Collapsin Response Mediator Protein 2 Ameliorates Disease Severity in a Mouse Model of Multiple Sclerosis. ASN Neuro 2019;11:1759091419892090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Moutal A, Khanna R. Unconventional Signaling by Extracellular CRMP2: Possible Role as an Atypical Neurotransmitter? Neuroscience 2018;376:224–226. [DOI] [PubMed] [Google Scholar]
- [45].Moutal A, Luo S, Largent-Milnes TM, Vanderah TW, Khanna R. Cdk5-mediated CRMP2 phosphorylation is necessary and sufficient for peripheral neuropathic pain. Neurobiol Pain 2019;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Moutal A, Sun L, Yang X, Li W, Cai S, Luo S, Khanna R. CRMP2-Neurofibromin Interface Drives NF1-related Pain. Neuroscience 2018;381:79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Moutal A, Wang Y, Yang X, Ji Y, Luo S, Dorame A, Bellampalli SS, Chew LA, Cai S, Dustrude ET, Keener JE, Marty MT, Vanderah TW, Khanna R. Dissecting the role of the CRMP2-neurofibromin complex on pain behaviors. Pain 2017;158(11):2203–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Moutal A, White KA, Chefdeville A, Laufmann RN, Vitiello PF, Feinstein D, Weimer JM, Khanna R. Dysregulation of CRMP2 Post-Translational Modifications Drive Its Pathological Functions. Molecular neurobiology 2019;56(10):6736–6755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Moutal A, Yang X, Li W, Gilbraith KB, Luo S, Cai S, Francois-Moutal L, Chew LA, Yeon SK, Bellampalli SS, Qu C, Xie JY, Ibrahim MM, Khanna M, Park KD, Porreca F, Khanna R. CRISPR/Cas9 editing of Nf1 gene identifies CRMP2 as a therapeutic target in neurofibromatosis type 1-related pain that is reversed by (S)-Lacosamide. Pain 2017;158(12):2301–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Nam Y, Kim JH, Kim JH, Jha MK, Jung JY, Lee MG, Choi IS, Jang IS, Lim DG, Hwang SH, Cho HJ, Suk K. Reversible Induction of Pain Hypersensitivity following Optogenetic Stimulation of Spinal Astrocytes. Cell Rep 2016;17(11):3049–3061. [DOI] [PubMed] [Google Scholar]
- [51].Price TJ, Basbaum AI, Bresnahan J, Chambers JF, De Koninck Y, Edwards RR, Ji RR, Katz J, Kavelaars A, Levine JD, Porter L, Schechter N, Sluka KA, Terman GW, Wager TD, Yaksh TL, Dworkin RH. Transition to chronic pain: opportunities for novel therapeutics. Nat Rev Neurosci 2018;19(7):383–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Ropert N, Miles R, Korn H. Characteristics of miniature inhibitory postsynaptic currents in CA1 pyramidal neurones of rat hippocampus. J Physiol 1990;428:707–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Scanziani M, Capogna M, Gahwiler BH, Thompson SM. Presynaptic inhibition of miniature excitatory synaptic currents by baclofen and adenosine in the hippocampus. Neuron 1992;9(5):919–927. [DOI] [PubMed] [Google Scholar]
- [54].Smith KM, Ross SE. Making connections: recent advances in spinal cord dorsal horn circuitry. Pain 2020;161 Suppl 1:S122–S126. [DOI] [PubMed] [Google Scholar]
- [55].Thompson SM, Capogna M, Scanziani M. Presynaptic inhibition in the hippocampus. Trends Neurosci 1993;16(6):222–227. [DOI] [PubMed] [Google Scholar]
- [56].Todd AJ. Neuronal circuitry for pain processing in the dorsal horn. Nat Rev Neurosci 2010;11(12):823–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Waxman SG, Zamponi GW. Regulating excitability of peripheral afferents: emerging ion channel targets. Nature neuroscience 2014;17(2):153–163. [DOI] [PubMed] [Google Scholar]
- [58].Wilson SM, Brittain JM, Piekarz AD, Ballard CJ, Ripsch MS, Cummins TR, Hurley JH, Khanna M, Hammes NM, Samuels BC, White FA, Khanna R. Further insights into the antinociceptive potential of a peptide disrupting the N-type calcium channel-CRMP-2 signaling complex. Channels (Austin) 2011;5(5):449–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Yu J, Moutal A, Dorame A, Bellampalli SS, Chefdeville A, Kanazawa I, Pham NYN, Park KD, Weimer JM, Khanna R. Phosphorylated CRMP2 Regulates Spinal Nociceptive Neurotransmission. Molecular neurobiology 2019;56(7):5241–5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Zeisel A, Hochgerner H, Lonnerberg P, Johnsson A, Memic F, van der Zwan J, Haring M, Braun E, Borm LE, La Manno G, Codeluppi S, Furlan A, Lee K, Skene N, Harris KD, Hjerling-Leffler J, Arenas E, Ernfors P, Marklund U, Linnarsson S. Molecular Architecture of the Mouse Nervous System. Cell 2018;174(4):999–1014 e1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Zhang J, Zhao B, Zhu X, Li J, Wu F, Li S, Gong X, Cha C, Guo G. Phosphorylation and SUMOylation of CRMP2 regulate the formation and maturation of dendritic spines. Brain research bulletin 2018;139:21–30. [DOI] [PubMed] [Google Scholar]
- [62].Zhang Y, Sloan SA, Clarke LE, Caneda C, Plaza CA, Blumenthal PD, Vogel H, Steinberg GK, Edwards MS, Li G, Duncan JA 3rd, Cheshier SH, Shuer LM, Chang EF, Grant GA, Gephart MG, Barres BA. Purification and Characterization of Progenitor and Mature Human Astrocytes Reveals Transcriptional and Functional Differences with Mouse. Neuron 2016;89(1):37–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Zhou HY, Chen SR, Pan HL. Targeting N-methyl-D-aspartate receptors for treatment of neuropathic pain. Expert review of clinical pharmacology 2011;4(3):379–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Zhou LJ, Peng J, Xu YN, Zeng WJ, Zhang J, Wei X, Mai CL, Lin ZJ, Liu Y, Murugan M, Eyo UB, Umpierre AD, Xin WJ, Chen T, Li M, Wang H, Richardson JR, Tan Z, Liu XG, Wu LJ. Microglia Are Indispensable for Synaptic Plasticity in the Spinal Dorsal Horn and Chronic Pain. Cell Rep 2019;27(13):3844–3859 e3846. [DOI] [PMC free article] [PubMed] [Google Scholar]