Figure 1.
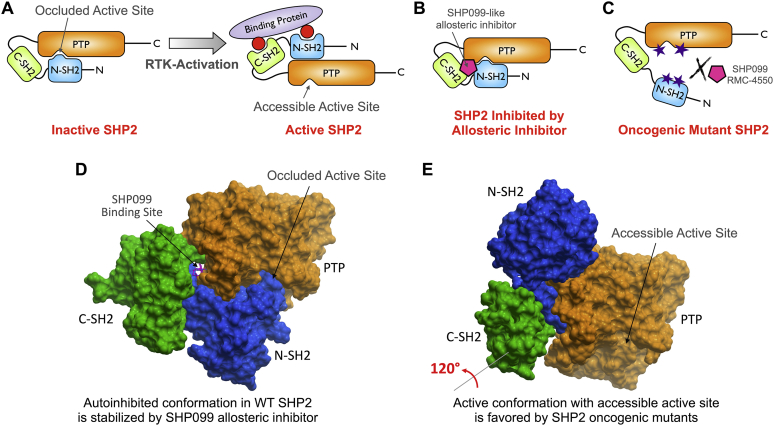
SHP2 regulation, inhibition, and oncogenic mutations.A, model of SHP2 activation. Receptor tyrosine kinase (RTK) activation leads to SHP2 recruitment by tyrosine phosphorylated motifs via its two SH2 domains, resulting in a conformational switch from the “closed” autoinhibited to the “open” active conformation. B, model of SHP2 inhibition by allosteric inhibitor, such as SHP099. These “molecular glue”–type compounds stabilize the SHP2 inactive closed conformation. C, model of the common mechanism of SHP2 oncogenic variant activation. A single amino-acid mutation at the N-SH2/PTP domain interface prevents the intramolecular binding of the two domains, resulting in a constitutively active SHP2. Allosteric inhibitors such as SHP099 and RMC-4550 are not able to bind the open conformation preferred by the SHP2 gain-of-function mutants. D, crystal structure of WT SHP2 representing the closed and autoinhibited conformation as described in (A) (Protein Data Bank ID: 5EHR). N-SH2 domain, blue; C-SH2 domain, green; PTP domain, orange; the allosteric inhibitor SHP099 (magenta, stick representation) binds to a channel formed by the PTP, N-SH2, and C-SH2 domains and stabilizes the inactive conformation as described in B. E, crystal structure of the SHP2 E76K gain-of-function mutant in the open and active conformation (Protein Data Bank ID: 6CRF). To adopt this conformation, the C-SH2 domain rotates by ∼120° and thereby translocates the N-SH2 domain away from the active site. SHP099-like allosteric inhibitors cannot effectively bind this active conformation as described in C. C-SH2, C-terminal SH2; N-SH2, N-terminal SH2 domain; PTP, protein tyrosine phosphatase; SH2, Src-homology 2; SHP2, Src-homology 2 domain–containing phosphatase 2.