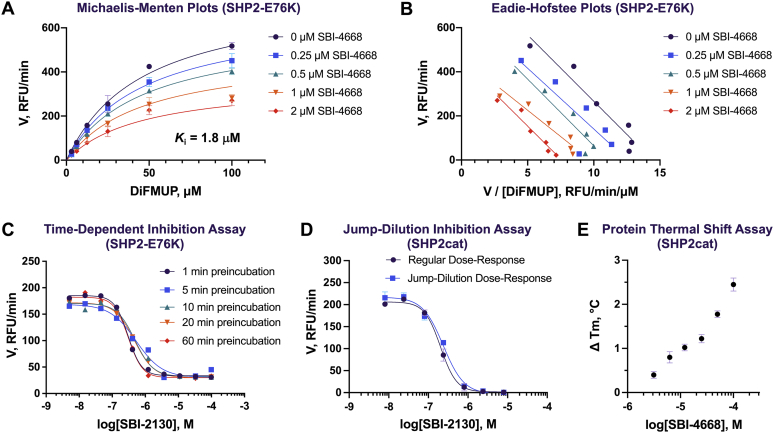
Figure 3.
Mechanism of action, inhibition, and binding studies of SHP2 inhibitors.A, Michaelis–Menten kinetic studies for the SHP2 inhibitor SBI-4668 with SHP2-E76K. Plots show the initial rates (V) at various substrates (DiFMUP) and inhibitor concentrations fitted to the Michaelis–Menten equation for noncompetitive inhibition. Relative fluorescence units per minute (RFU/min) are represented as mean ± SD (n = 3). B, Eadie–Hofstee plots of the Michaelis–Menten kinetic studies with compound SBI-4668 from A. C, dose–response curves for SBI-2130 with SHP2-E76K after various preincubation times of inhibitor with SHP2. RFU/min are represented as mean ± SD (n = 3). No time-dependent inhibition was observed as demonstrated by the similar potency for the various time points. D, dose–response curves for SBI-2130 with SHP2cat with or without a 10× inhibitor/protein preincubation and jump dilution. Identical IC50 curves indicate that SBI-2130 is a reversible inhibitor. RFU/min are represented as mean ± SD (n = 4). E, dose-dependent binding of SBI-4668 to SHP2cat in a protein thermal shift (PTS) assay. Thermal stabilization of SHP2 by SBI-4668 is shown by the increase in the SHP2 melting temperature (ΔTm) compared with vehicle control (DMSO). ΔTm values are represented as mean ± SD (n = 4). DiFMUP, 6,8-difluoro-4-methylumbelliferyl phosphate; DMSO, dimethyl sulfoxide; SHP2, Src-homology 2 domain–containing phosphatase 2; SHP2cat, SHP2 catalytic domain.