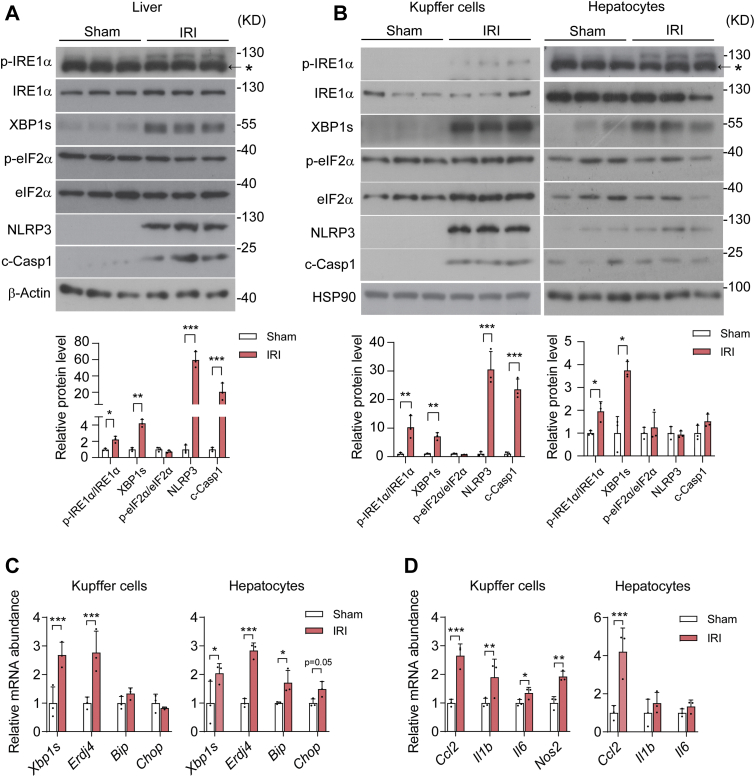
Figure 1.
The IRE1α pathway is activated in parallel with the NLRP3 inflammasome in Kupffer cells upon hepatic ischemia/reperfusion. Male C57BL6 mice were subjected to 60 min of partial hepatic warm ischemia followed by 6 h of reperfusion to induce hepatic ischemia/reperfusion injury (IRI), and sham-treated mice were used as control (n = 3 per group). A, immunoblot analysis of the indicated proteins in whole liver lysates. The asterisk indicates the nonspecific band detected by the phospho-IRE1α antibody. β-Actin was used as the loading control. Shown also is quantification of IRE1α and eIF2α phosphorylation as well as XBP1s, NLRP3, and c-Casp1 protein levels. B, immunoblot analysis of the indicated proteins in cell lysates of freshly isolated Kupffer cells and hepatocytes from livers of the IRI and sham control group. The asterisk indicates the nonspecific band detected in hepatocytes by the phospho-IRE1α antibody. HSP90 was used as the loading control. Shown also is quantification of IRE1α and eIF2α phosphorylation as well as the indicated proteins. C, quantitative RT-PCR (qRT-PCR) analysis of mRNA abundance of the indicated UPR genes in freshly isolated Kupffer cells and hepatocytes. D, qRT-PCR analysis of mRNA abundance of the indicated inflammatory genes in freshly isolated Kupffer cells and hepatocytes. Data are shown as the mean ± SD. ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001 by unpaired two-tailed Student's t test. NLRP3, nucleotide-binding domain, leucine-rich repeat containing protein 3; UPR, unfolded protein response.