figs3.
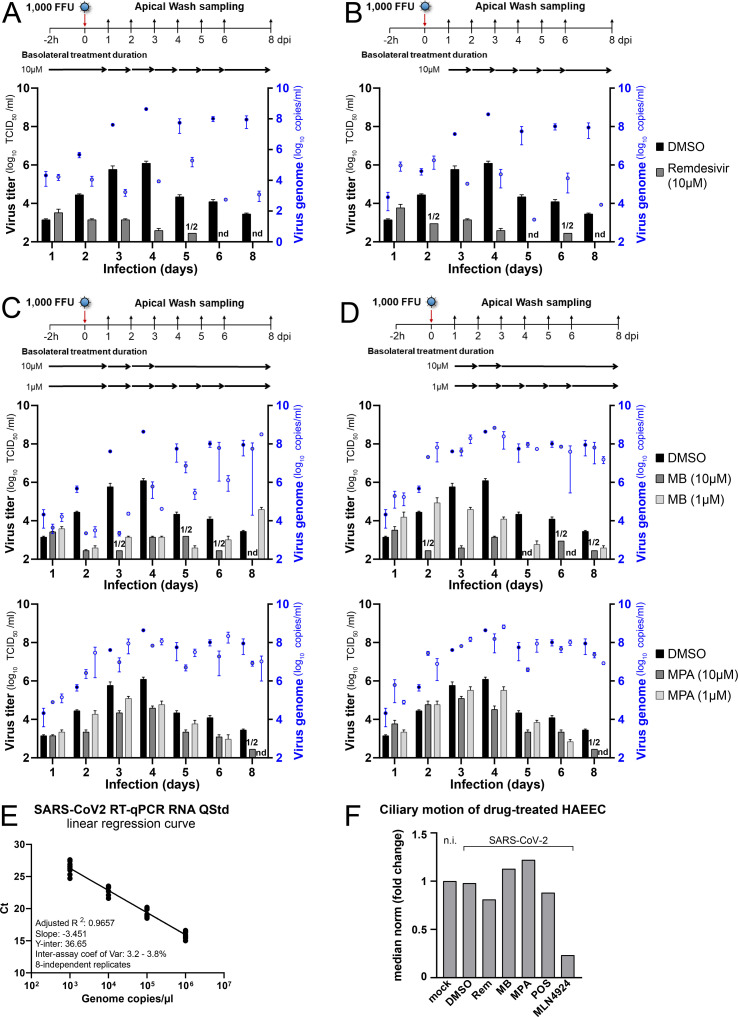
MB, MPA inhibit SARS-CoV-2 infection of nasal HAEEC. Viral titer (TCID50/ml) readout (left y-axis) are those shown in Fig. 5A, complemented here with the corresponding viral genome copy numbers (blue right y-axis). Data represent the mean+/-SEM of two independent replicates. Inserts treated in a pre-infection regimen, starting at 2h prior to virus inoculation (left) or in a post-infection regimen starting at 1d pi (right) with Remdesivir (A-B), MB (C), and with MPA (D). Not determined (nd) indicates virus titers below 2.4 log10 TCID50/ml in the two replicates, and (1/2) indicates virus titers below 2.4 log10 TCID50/ml in one replicate. E) The SARS-CoV-2 RT-qPCR inter-assay reproducibility is represented with the linear regression curve generated from the Ct values and the copy numbers (106 to 103 copies per μl) of the RNA quantitative standards in eight independent replicates. The coefficient of correlation (CV) was calculated for each quantitative point according to the formula: %CV = (SD / Ct mean)×100. F) Effect of drug treatment on ciliary motion of SARS-CoV-2 infected nasal HAEEC. Transmitted light movies were recorded at 5d pi (MLN4924, Posaconazole), 6d pi (DMSO, Remdesivir, MB, MPA), or 6d in culture in absence of virus or drug (mock). Ciliary motion was assessed by computing the median norm of the optical flow per frame. Fold changes are normalized to mock. Panel refers to Supplemental Movies 1-7.