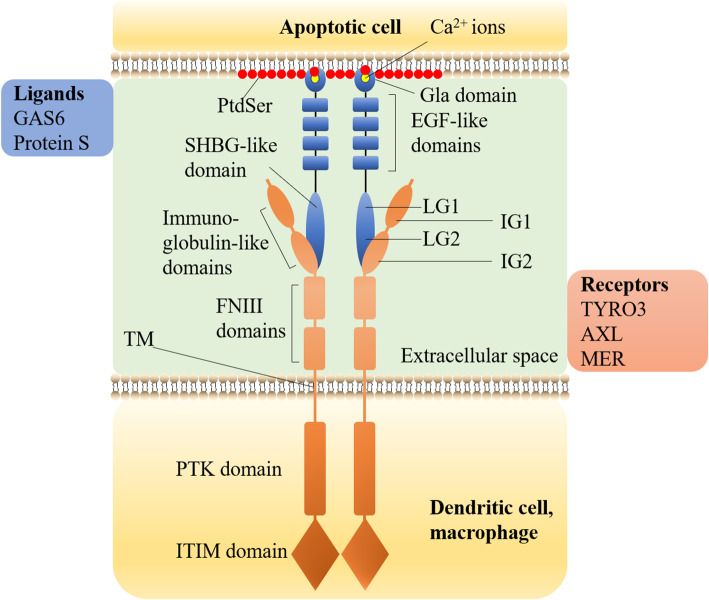
Fig. 3.
The Structure of TAM, GAS6/Protein S and how they bind to PtdSer. TAM is a single transmembrane receptor (the orange part in the figure). From N-terminal to C-terminal (from top to bottom in the figure), there are three parts:1, the extracellular domain, including two IG domains (IG1, IG2) and two FNIII domains; 2, one single TM; 3, the intracellular domain, including one conserved PTK domain, one autophosphorylation site, and one ITIM domain [23–27]. The intracellular PTK domain of TAM is the functional domain. TYRO3, AXL, and MER of TAM family all have phosphorylation and autophosphorylation sites, which can activate the corresponding signaling pathways by phosphorylation of downstream target proteins. TAM does not directly bind to the PtdSer exposed on the cell surface but relies on GAS6 or PROS1 as a ligand [28]. GAS6/PROS1 consists of three parts (the blue part in the figure). From N-terminal to C-terminal (from top to bottom in the figure), there are N-terminal GLA domain, four EH -like domains, and C-terminal SHBG domain [7, 29, 30]. The TAM receptor binds to the C-terminal SHBG domain of GAS6/PROS1 through two N-terminal IG1 and IG2 in the extracellular domain. SHBG domain includes two tandem LG domains (LG1, LG2). The binding eventually leads to the activation of TAM receptor tyrosine kinase. GAS6/PROS1 binds to the PtdSer exposed outside the cell through the N-terminal GLA domain. With the assistance of vitamin K, the structure of the GLA domain can be stabilized through the binding of about 7 essential Ca2+ ions (yellow ball at the top in the figure) [7, 31–34]. The specific mechanism of interaction between the GLA domain and PtdSer will be introduced in section 4.2