Abstract
Background
Child and adolescent overweight and obesity have increased globally, and are associated with short‐ and long‐term health consequences.
Objectives
To assess the efficacy of diet, physical activity and behavioural interventions delivered to parents only for the treatment of overweight and obesity in children aged 5 to 11 years.
Search methods
We performed a systematic literature search of databases including the Cochrane Library, MEDLINE, EMBASE, PsycINFO, CINAHL and LILACS as well trial registers. We checked references of identified trials and systematic reviews. We applied no language restrictions. The date of the last search was March 2015 for all databases.
Selection criteria
We selected randomised controlled trials (RCTs) of diet, physical activity and behavioural interventions delivered to parents only for treating overweight or obesity in children aged 5 to 11 years.
Data collection and analysis
Two review authors independently assessed trials for risk of bias and evaluated overall study quality using the GRADE instrument. Where necessary, we contacted authors for additional information.
Main results
We included 20 RCTs, including 3057 participants. The number of participants ranged per trial between 15 and 645. Follow‐up ranged between 24 weeks and two years. Eighteen trials were parallel RCTs and two were cluster RCTs. Twelve RCTs had two comparisons and eight RCTs had three comparisons. The interventions varied widely; the duration, content, delivery and follow‐up of the interventions were heterogeneous. The comparators also differed. This review categorised the comparisons into four groups: parent‐only versus parent‐child, parent‐only versus waiting list controls, parent‐only versus minimal contact interventions and parent‐only versus other parent‐only interventions.
Trial quality was generally low with a large proportion of trials rated as high risk of bias on individual risk of bias criteria.
In trials comparing a parent‐only intervention with a parent‐child intervention, the body mass index (BMI) z score change showed a mean difference (MD) at the longest follow‐up period (10 to 24 months) of ‐0.04 (95% confidence interval (CI) ‐0.15 to 0.08); P = 0.56; 267 participants; 3 trials; low quality evidence. In trials comparing a parent‐only intervention with a waiting list control, the BMI z score change in favour of the parent‐only intervention at the longest follow‐up period (10‐12 months) had an MD of ‐0.10 (95% CI ‐0.19 to ‐0.01); P = 0.04; 136 participants; 2 trials; low quality evidence. BMI z score change of parent‐only interventions when compared with minimal contact control interventions at the longest follow‐up period (9 to 12 months) showed an MD of 0.01 (95% CI ‐0.07 to 0.09); P = 0.81; 165 participants; 1 trial; low quality evidence. There were few similarities between interventions and comparators across the included trials in the parent‐only intervention versus other parent‐only interventions and we did not pool these data. Generally, these trials did not show substantial differences between their respective parent‐only groups on BMI outcomes.
Other outcomes such as behavioural measures, parent‐child relationships and health‐related quality of life were reported inconsistently. Adverse effects of the interventions were generally not reported, two trials stated that there were no serious adverse effects. No trials reported on all‐cause mortality, morbidity or socioeconomic effects.
All results need to be interpreted cautiously because of their low quality, the heterogeneous interventions and comparators, and the high rates of non‐completion.
Authors' conclusions
Parent‐only interventions may be an effective treatment option for overweight or obese children aged 5 to 11 years when compared with waiting list controls. Parent‐only interventions had similar effects compared with parent‐child interventions and compared with those with minimal contact controls. However, the evidence is at present limited; some of the trials had a high risk of bias with loss to follow‐up being a particular issue and there was a lack of evidence for several important outcomes. The systematic review has identified 10 ongoing trials that have a parent‐only arm, which will contribute to future updates. These trials will improve the robustness of the analyses by type of comparator, and may permit subgroup analysis by intervention component and the setting. Trial reports should provide adequate details about the interventions to be replicated by others. There is a need to conduct and report cost‐effectiveness analyses in future trials in order to establish whether parent‐only interventions are more cost‐effective than parent‐child interventions.
Plain language summary
Parent‐only interventions for childhood overweight or obesity in children aged 5 to 11 years
Review question
How effective are diet, physical activity and behavioural interventions delivered to parents only in reducing the weight of overweight and obese children?
Background
Across the world more children are becoming overweight and obese. These children are more likely to suffer from health problems as children and in later life. Parents can play an important role in determining what their children eat. More information is needed about whether helping parents to make changes to their family's diet and lifestyle will treat this problem.
Study characteristics
We found 20 randomised controlled trials (clinical studies where people are randomly put into one of two or more treatment groups) comparing diet, physical activity and behavioural (where habits are changed or improved) treatments (interventions) to a variety of control groups (who did not receive treatment) delivered to parents only of 3057 children aged 5 to 11 years. There were few similarities between the trials in the nature and types of interventions used. We grouped the trials by the type of comparisons. Our systematic review reported on the effects of the parent‐only interventions compared with parent and child interventions, waiting list controls (where the intervention was delayed until the end of the trial), other interventions with only minimal information or contact and other types of parent‐only interventions. The children in the included trials were monitored (called follow‐up) for between six months and two years. This evidence is up to date as of March 2015.
Key results
The most reported outcome was the body mass index (BMI). This is a measure of body fat and is calculated by dividing weight (in kilograms) by the square of the body height measured in metres (kg/m2). The studies measured BMI in ways that took account of gender, weight and height as the children grew older (such as the BMI z score and the BMI percentile).
When compared with a waiting list control, there was limited evidence that parental interventions helped to reduce BMI. In looking at the longest follow‐up periods of the included trials, we did not find firm evidence of an advantage or disadvantage of parent‐only interventions when compared with either parent and child interventions, or when compared with limited information. Our review found very little information about how different types of parental interventions compared. No trial reported on death from any cause, illness or socioeconomic effects (such as whether parent‐only interventions are lower in costs compared with parent and child interventions). Two trials reported no serious side effects and the rest of the trials did not report whether side effects occurred or not. Information on parent‐child relationships and health‐related quality of life was rarely reported.
Quality of the evidence
The overall quality of the evidence was low, mainly because there were just a few trials per measurement or the number of the included children was small. In addition, many children left the trials before they had finished.
Summary of findings
Summary of findings for the main comparison. Parent‐only interventions versus parent‐child interventions for childhood overweight or obesity.
| Parent‐only interventions vs. parent‐child interventions for childhood overweight or obesity | ||||||
|
Population: children with overweight or obesity Settings: outpatients; community/university Intervention: parent‐only interventions Comparison: parent‐child interventions | ||||||
| Outcomes | Parent‐child | Parent‐only | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | Comments |
|
BMI z score change (x * SD) Follow‐up: 40‐104 weeks |
The mean BMI z score change ranged across control groups from ‐0.16 to ‐0.24 | The mean BMI z score change in the intervention groups was 0.04 lower (0.15 lower to 0.08 higher) | ‐ | 267 (3) | ⊕⊕⊝⊝ lowa | Lower scores indicate improved weight loss |
| Adverse events | See comment | See comment | See comment | See comment | See comment | No trials reported adverse events |
| Health‐related quality of life | See comment | See comment | See comment | See comment | See comment | No trials reported health‐related quality of life |
| All‐cause mortality | See comment | See comment | See comment | See comment | See comment | No trials reported all‐cause mortality |
| Morbidity | See comment | See comment | See comment | See comment | See comment | No trials reported morbidity |
| Parent‐child relationship or assessment of parenting | See comment | See comment | See comment | See comment | See comment | No trials reported outcomes assessing parent‐child relationships or an assessment of parenting |
| Socioeconomic effects | See comment | See comment | See comment | See comment | See comment | No trials reported socioeconomic effects |
| *The basis for the assumed risk (e.g. the median control group risk across trials) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
BMI: body mass index; CI: confidence interval; SD: standard deviation. GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
"A BMI z score or standard deviation score indicates how many units (of the standard deviation) a child's BMI is above or below the average BMI value for their age group and sex. For instance, a z score of 1.5 indicates that a child' is 1.5 standard deviations above the average value, and a z score of ‐1.5 indicates a child is 1.5 standard deviations below the average value" (Noo NHS 2011).
aDowngraded by one level because of serious risk of attrition bias and one level for serious imprecision (see Appendix 9).
Summary of findings 2. Parent‐only interventions versus waiting list control for childhood overweight or obesity.
| Parent‐only interventions vs. waiting list control for childhood overweight or obesity | ||||||
|
Population: children with overweight or obesity Settings: outpatients; community Intervention: parent‐only interventions Comparison: waiting list control | ||||||
| Outcomes | Waiting list | Parent‐only | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | Comments |
|
BMI z score change (x * SD) Follow‐up: 40‐48 weeks |
The mean BMI z score change ranged across control groups from ‐0.13 to 0.02 | The mean BMI z score change in the intervention groups was 0.1 lower (0.19 lower to 0.01 lower) | ‐ | 136 (2) | ⊕⊕⊝⊝ lowa | Lower scores indicate improved weight loss |
| Adverse events | See comment | See comment | See comment | See comment | See comment | No trials reported adverse events |
| Health‐related quality of life | See comment | See comment | See comment | See comment | See comment | No trials reported health‐related quality of life |
| All‐cause mortality | See comment | See comment | See comment | See comment | See comment | No trials reported all‐cause mortality |
| Morbidity | See comment | See comment | See comment | See comment | See comment | No trials reported morbidity |
|
Parent‐child relationship or assessment of parenting (parenting scale (PS), 30 items, scored from 1 to 7; lower scores indicate more effective parental discipline practices) Follow‐up: 12 weeks |
The mean PS score for the control group was 3.4 | The mean PS score in the intervention group was 0.6 points lower | ‐ | 101 (1) | ⊕⊕⊝⊝ lowa | ‐ |
| Socioeconomic effects | See comment | See comment | See comment | See comment | See comment | No trials reported socioeconomic effects |
| *The basis for the assumed risk (e.g. the median control group risk across trials) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
BMI: body mass index; CI: confidence interval; PS: parenting scale; SD: standard deviation. GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
"A BMI z score or standard deviation score indicates how many units (of the standard deviation) a child's BMI is above or below the average BMI value for their age group and sex. For instance, a z score of 1.5 indicates that a child' is 1.5 standard deviations above the average value, and a z score of ‐1.5 indicates a child is 1.5 standard deviations below the average value" (Noo NHS 2011).
aDowngraded by one level because of serious risk of attrition bias and one level for serious imprecision (see Appendix 9).
Summary of findings 3. Parent‐only interventions versus minimal contact control for childhood overweight or obesity.
| Parent‐only interventions vs. minimal contact control for childhood overweight or obesity | ||||||
|
Population: children with overweight or obesity Settings: outpatients Intervention: parent‐only interventions Comparison: minimal contact control | ||||||
| Outcomes | Minimal contact | Parent‐only | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | Comments |
|
BMI z score change (x * SD) Follow‐up: 52 weeks |
The mean BMI z score change ranged across control groups from ‐0.06 to ‐0.06 | The mean BMI z score change in the intervention group was 0.01 lower (‐0.07 lower to 0.09 higher) | ‐ | 165 (1) | ⊕⊕⊝⊝ lowa | Lower scores indicate improved weight loss |
| Adverse events | See comment | See comment | See comment | See comment | See comment | No trials reported adverse events |
|
Health‐related quality of life (Pediatric Health‐Related Quality of Life, scale from 0 to 100; higher scores indicate better HRQoL) Follow‐up: 24 weeks) |
See comment | See comment | See comment | 93 (1) | See comment | No data were presented ('"no improvements in health‐related quality of life") |
| All‐cause mortality | See comment | See comment | See comment | See comment | See comment | No trials reported all‐cause mortality |
| Morbidity | See comment | See comment | See comment | See comment | See comment | No trials reported morbidity |
|
Parent‐child relationship or assessment of parenting (Child Feeding Questionnaire subscale parental concern (total of 7 subscales), score range 3‐15; higher scores indicate greater parental concern) Follow‐up: 12 weeks |
The mean parent concern score was 4.7 in the control group | The mean parent concern score in the intervention group was 0.1 lower. | ‐ | 93 (1) | ⊕⊕⊝⊝ lowa | ‐ |
| Socioeconomic effects | See comment | See comment | See comment | See comment | See comment | No trials reported socioeconomic effects |
| *The basis for the assumed risk (e.g. the median control group risk across trials) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
BMI: body mass index; CI: confidence interval; HRQoL: health‐related quality of life; SD: standard deviation. GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
"A BMI z score or standard deviation score indicates how many units (of the standard deviation) a child's BMI is above or below the average BMI value for their age group and sex. For instance, a z score of 1.5 indicates that a child' is 1.5 standard deviations above the average value, and a z score of ‐1.5 indicates a child is 1.5 standard deviations below the average value" (Noo NHS 2011).
aDowngraded by one level because of serious risk of attrition bias and one level for serious imprecision (see Appendix 9).
Summary of findings 4. Parent‐only interventions versus parent‐only interventions for childhood overweight or obesity.
| Parent‐only interventions vs. parent‐only interventions for childhood overweight or obesity | ||||||
|
Population: children with overweight or obesity Settings: outpatients; university + primary care Intervention: parent‐only interventions Comparison: parent‐only interventions | ||||||
| Outcomes | Parent‐only | Parent‐only | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | Comments |
|
BMI z score change (x * SD) Follow‐up: 12‐24 months |
See comment | See comment | See comment | 467 (5) | ⊕⊕⊝⊝ lowa | No meta‐analysis because of little consistency between trial interventions and comparators; there were no substantial differences between different parent‐only interventions |
| Adverse events | See comment | See comment | See comment | See comment | See comment | Two trials reported that there were no serious adverse events (Raynor 2012a; Raynor 2012b) |
| Health‐related quality of life | See comment | See comment | See comment | See comment | See comment | No trials reported health‐related quality of life |
| All‐cause mortality | See comment | See comment | See comment | See comment | See comment | No trials reported all‐cause mortality |
| Morbidity | See comment | See comment | See comment | See comment | See comment | No trials reported morbidity |
|
Parent‐child relationship or assessment of parenting (Alabama Parenting Questionnaire, 35 items; higher scores indicate improvement) Follow‐up: 24 months |
See comment | See comment | See comment | 106 (1) | See comment | 1 study assessed parent‐child relationship or assessment of parenting but there were no data for comparisons between intervention groups provided |
| Socioeconomic effects | See comment | See comment | See comment | See comment | See comment | No trials reported socioeconomic effects |
| *The basis for the assumed risk (e.g. the median control group risk across trials) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
BMI: body mass index; CI: confidence interval; SD: standard deviation. GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
"A BMI z score or standard deviation score indicates how many units (of the standard deviation) a child's BMI is above or below the average BMI value for their age group and sex. For instance, a z score of 1.5 indicates that a child' is 1.5 standard deviations above the average value, and a z score of ‐1.5 indicates a child is 1.5 standard deviations below the average value" (Noo NHS 2011).
aDowngraded by one level because of serious risk of attrition bias and one level for serious imprecision (see Appendix 9)
Background
The prevalence of overweight and obese children and adolescents has increased throughout the world, presenting a global public health crisis (Ng 2014; WHO 2015a). Although once considered to be a condition affecting only developed countries, rates of paediatric overweight and obesity have started to rise dramatically in some developing countries (Wang 2012). Using the International Obesity Task Force (IOTF) standard definition, the age‐standardised prevalence of overweight and obesity in children and adolescents has increased in both developed and developing countries since the mid‐1980s (Cole 2000). In 2013, the prevalence of overweight and obese children and adolescents in developed countries was estimated at 23.8% (95% confidence interval (CI) 22.9 to 24.7)) for boys and 22.6% (95% CI 21.7 to 23.6) for girls. In developing countries, the prevalence was estimated as 12.9% (95% CI 12.3 to 13.5) for boys and 13.4% (95% CI 13.0 to 13.9) for girls (Ng 2014). Very young children are also affected. In 2010, de Onis 2010 used the World Health Organization (WHO) growth standards (WHO 2015b) to estimate that over 42 million children under five years of age were overweight or obese, with approximately 35 million of these children living in developing countries.
Inequalities in overweight and obesity prevalence have also been documented. Generally, socioeconomically disadvantaged children in developed countries (Knai 2012; Shrewsbury 2008), and children of higher socioeconomic status in developing countries (Lobstein 2004; Wang 2012), are at greater risk of becoming overweight. However, this relationship may vary by population demographics (e.g. age, gender, ethnicity), and environment (e.g. country, urbanisation) (Wang 2012). The prevalence of obesity varies by ethnicity, with large data sets showing substantial ethnic variation in English (HSCIC 2015), American (Freedman 2006; Skinner 2014), and New Zealand (Rajput 2014) child populations.
While there is some evidence that the rate of increase in paediatric obesity may be slowing in some developed countries, current levels remain too high, and continue to rise in many developing countries (Olds 2011; Rokholm 2010). However, an additional concern in some developed countries such as the USA (Kelly 2013; Skinner 2014), and England (CMO 2012; Ells 2015), is the rise in severe paediatric obesity. While the IOTF published an international definition for severe paediatric (morbid) obesity in 2012 (Cole 2012), often severe obesity prevalence is reported using country‐specific cut‐off points making international comparisons difficult. However, data from the USA (Skinner 2014), and England (Ells 2015), have shown that the prevalence of severe paediatric obesity varies by socioeconomic status and ethnicity, and may result in a greater risk of adverse cardio‐metabolic events and severe obesity in adulthood (Kelly 2013).
Description of the condition
Childhood overweight and obesity results from an accumulation of excess body fat, and can increase the risk of both short‐ and longer‐term health consequences. Numerous obesity‐related co‐morbidities can develop during childhood, which include muscular skeletal complaints (Paulis 2014); cardiovascular risk factors such as hypertension, insulin resistance and hyperlipidaemia (Reilly 2003), even in very young children (Bocca 2013); and conditions such as such as sleep apnoea (Narang 2012), asthma (Egan 2013), liver disease, and type 2 diabetes (Daniels 2009; Lobstein 2004). The condition can also affect psychosocial well‐being, with obese young people susceptible to reduced self esteem and health‐related quality of life (Griffiths 2010), and stigmatisation (Puhl 2007; Tang‐Peronard 2008). Evidence also shows that childhood obesity can track into adulthood (Parsons 1999; Singh 2008; Whitaker 1997), and is therefore associated with an increased risk of ill health later in life (Reilly 2011).
Description of the intervention
Given the serious implications associated with childhood and adolescent obesity, effective treatment is imperative. While the fundamental principles of weight management in children and adolescents are the same as adults (i.e. reduced energy intake and increased energy expenditure), the primary aim of treatment (i.e. weight reduction or deceleration of weight gain) and the most suitable intervention approach varies, and is dependent on the child's age and degree of excess weight, among other considerations.
Family‐based interventions combining dietary, physical activity and behavioural components are effective and are considered as the current best practice in the treatment of childhood obesity in children under 12 years of age (Oude Luttikhuis 2009). However, interventions that involve the whole family can be costly, especially with parents and children in separate groups and when not running at full capacity (Upton 2012). Therefore, increased attention is being paid to the possibility of parent‐focused interventions.
Parents have been defined as the 'agents of change' for intervening with children under 12 years of age who are obese (Golan 2004). Several interventions have been developed where parents are targeted solely for the treatment of their child's obesity, thereafter referred to as 'parent‐only' interventions, in which the child is not involved directly with the intervention. Parent‐only interventions vary both by type (e.g. based on parenting courses, cognitive behavioural therapy, behaviour change) and by setting (e.g. community, clinic based).
Adverse effects of the intervention
It is not anticipated that parent‐only interventions will lead to adverse outcomes. However, as with all obesity treatment interventions in children and young people, potential adverse effects should be considered, including effects on linear growth, eating disorders and psychological well‐being.
How the intervention might work
The home environment is important in the aetiology of childhood obesity, with parents playing a large role in food choice and physical activity for their children. In surveys in the US, Wansink estimated that the 'nutritional gatekeeper' (who buys and cooks the food) controls 72% of the food eaten by children, both within and outside the home (Wansink 2006). One systematic review by Clark et al. showed that a high level of parental restriction of snack foods is associated with increased energy intake and weight gain in children (Clark 2007). In contrast, 'covert' control of children's food intake by controlling the home eating environment to limit exposure to unhealthy foods (i.e. not buying unhealthy foods) lows the intake of unhealthy snacks when compared with 'overt' control (i.e. buying the snacks but not allowing access) (Ogden 2006). In terms of physical activity, one systematic review showed that parental support is strongly associated with physical activity levels in children, albeit the influence of parental modelling by being physically active themselves was inconsistent (Gustafson 2006).
Poor family functioning, such as poor communication and high levels of conflict, is also associated with higher risk of obesity in children (Halliday 2014). Authoritative parenting style is associated with lower risk of obesity in children, when compared with other parenting styles (Sleddens 2011).
Due to the importance of the role of parents in the home environment and the importance of parenting styles and skills, parents have been defined as the 'agents of change' in the family for intervening with children under 12 years of age who are obese (Golan 2004). The importance of parents in the change process has led to a questioning of whether children need to be at the intervention. Parent‐only interventions aim to work by giving parents the responsibility for their family's eating and physical activity environment and by increasing parental capacity to implement the lifestyle changes. Trials have assessed whether parent‐only interventions are superior to or equivalent to parent‐child interventions, as well as comparisons with waiting list control.
Why it is important to do this review
The first version of this systematic review was published in 2003 and included analysis of childhood obesity treatment trials published up to July 2001 (Summerbell 2003). The second version was published in 2009 providing an update to the 2003 review (Oude Luttikhuis 2009).
To reflect the rapid growth in this field, the third update to this review has been split across six reviews focusing on the following treatment approaches: surgery; drugs; parent‐only interventions; diet, physical activity and behavioural interventions for young children aged 0 to 4 years; school children aged 5 to 11 years and adolescents aged 12 to 17 years.
The current review examined the effectiveness of interventions in which parents were targeted solely for the treatment of childhood obesity. This review built on two reviews in this area in which parent‐only interventions appeared to be as effective as interventions that adopted the traditional model where the parent and child were both involved in the intervention (Ewald 2014; Jull 2013). Faith 2012 revealed inconsistent evidence that greater parent and adult carer involvement was associated with better child outcomes. This review extended the evidence of effectiveness by including trials that compared parent‐only interventions with parent‐child interventions, waiting list controls, other interventions with only minimal information or contact and other types of parent‐only interventions. These trials were brought together to examine the effectiveness of parent‐only interventions for the treatment of childhood obesity. The review also intended to explore the impact of the type of parent‐only intervention (e.g. focusing on parenting, cognitive behavioural therapy, behaviour change) and the setting (e.g. community, clinic‐based, internet), to determine if any specific approach was more effective for the treatment of childhood obesity.
The results of this current review and other systematic reviews in this series provided information on which to underpin clinical guidelines and health policy on the treatment of childhood overweight or obesity.
Objectives
To assess the efficacy of diet, physical activity and behavioural interventions delivered to parents only for the treatment of overweight and obesity in children aged 5 to 11 years.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs).
Types of participants
Study groups consisted of children with a mean study age of 5 to 11 years at the commencement of the intervention.
Diagnostic criteria
We included overweight or obese children by any classification.
Types of interventions
We planned to investigate the following comparisons of intervention versus control/comparator.
Intervention
Any form of lifestyle intervention with a primary aim to treat overweight or obesity in children (any form of dietary, physical activity, behavioural therapy, or a combination of these delivered as single or multi‐component interventions) directed at the parents as the agents of change (i.e. interventions did not include their children).
Comparator
Usual care, a parent‐child intervention, child only intervention or an alternative concomitant therapy providing it was delivered in the intervention arm.
Concomitant interventions had to be the same in the intervention and comparator groups to establish fair comparisons.
Minimum duration of intervention/follow‐up
Duration of intervention/follow‐up had to be at least six months.
Exclusion criteria
We excluded critically ill children or children with a syndromic cause for their obesity (e.g. Prader‐Willi).
Types of outcome measures
Primary outcomes
Changes in body mass index (BMI) and body weight.
Adverse events.
Secondary outcomes
Health‐related quality of life and self esteem.
All‐cause mortality.
Morbidity.
Measures of body fat distribution.
Behaviour change.
Participants' views of the intervention.
Parent‐child relationship or assessment of parenting.
Socioeconomic effects by validated measures.
Method and timing of outcome measurement
Changes in BMI (kg/m2) and body weight (kg) measured at baseline, and at least at 6, 12 and 24 months.
Adverse events: defined as an adverse outcome that occurred during or after the intervention but was not necessarily caused by it, and measured at baseline, and at least at 6, 12 and 24 months.
Health‐related quality of life: evaluated by a validated instruments such as the Paediatric Quality of Life Inventory and measured at baseline, and at least at 6, 12 and 24 months.
All‐cause mortality: defined as any death that occurred during or after the intervention and measured at baseline, and at least at 6, 12 and 24 months
Morbidity: defined as illness or harm associated with the intervention and measured at baseline, and at least at 6, 12 and 24 months.
Measures of body fat distribution: defined using validated tools such as dual‐energy X‐ray absorptiometry (DXA), waist circumference, skin‐fold thickness, waist‐to‐hip ratio or bioelectrical impedance analysis and measured at baseline, and at least at 6, 12 and 24 months.
Behaviour change: defined as validated measures of diet and physical activity and measured at baseline, and at least at 6, 12 and 24 months.
Participants' views of the intervention: defined as documents accounts from participant feedback and measured at baseline, and at least at 6, 12 and 24 months.
Parent‐child relationship or assessment of parenting: evaluated by a validated instrument and measured at baseline, and at least at 6, 12 and 24 months.
Socioeconomic effects defined as a validated measure of socioeconomic status such as parental income or educational status and measured at baseline, and at least at 6, 12 and 24 months.
Summary of findings
We present a 'Summary of findings' table to report the following outcomes, listed according to priority.
Changes in BMI and body weight.
Adverse events.
Health‐related quality of life
All‐cause mortality.
Mobidity.
Parent‐dhild relationship or assessment of parenting.
Socioeconomic effects.
Search methods for identification of studies
Electronic searches
We searched the following sources from inception of each database to the specified date and placed no restrictions on the language of publication.
-
Cochrane Library:
Cochrane Database of Systematic Reviews (CDSR) (Issue 3, 10 March 2015).
Cochrane Central Register of Controlled Trials (CENTRAL) (Issue 3, 10 March 2015).
Database of Abstracts of Reviews of Effects (DARE) (Issue 1, 10 March 2015).
Health Technology Assessment (HTA) (Issue 1, 10 March 2015).
MEDLINE and MEDLINE In‐Process & Other Non‐Indexed Citations, 1946 to 10 March 2015.
EMBASE, 1974 to 10 March 2015.
PsycINFO, 1806 to 10 March 2015.
CINAHL (10 March 2015).
LILACS (10 March 2015).
ClinicalTrials.gov (10 March 2015).
-
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) Search Portal (http://apps.who.int/trialsearch/), which is a meta‐register of trials with links to several trial registers, that includes
Australian New Zealand Clinical Trials Registry (2 March 2015).
Chinese Clinical Trial Registry (2 March 2015).
ClinicalTrials.gov (2 March 2015).
EU Clinical Trials Register (EU‐CTR) (2 March 2015).
ISRCTN (International Standard Randomised Controlled Trial Number) Register (2 March 2015).
The Netherlands National Trial Register (2 March 2015).
Brazilian Clinical Trials Registry (2 February 2015).
Clinical Trials Registry ‐ India (2 March 2015).
Clinical Research Information Service ‐ Republic of Korea (3 March 2015).
Cuban Public Registry of Clinical Trials (3 March 2015).
German Clinical Trials Register (3 March 2015).
Iranian Registry of Clinical Trials (3 March 2015).
Japan Primary Registries Network (3 March 2015).
Pan African Clinical Trial Registry (3 March 2015).
Sri Lanka Clinical Trials Registry (2 March 2015).
Thai Clinical Trials Register (3 March 2015).
We continuously applied a MEDLINE (via Ovid) email alert service established by the Cochrane Metabolic and Endocrine Disorders (CMED) Group to identify newly published trials using the same search strategy as described for MEDLINE (for details on search strategies see Appendix 1). Should we have identified new trials for inclusion, we would have evaluated these, incorporated findings in our review and re‐submitted another review draft (Beller 2013).
Searching other resources
We tried to identify other potentially eligible trials or ancillary publications by searching the reference lists of retrieved included trials, (systematic) reviews, meta‐analyses and HTA reports.
Data collection and analysis
Selection of studies
Two review authors (two of RJ, LA, KR, EL, JC, WR, EM) independently scanned the abstract, title, or both, of every record retrieved, to determine which trials should be assessed further. We investigated all potentially relevant articles as full text. We resolved any discrepancies through consensus or recourse to a third review author (KR, EL, LA). Where resolution of a disagreement was not possible, we added the article to those 'awaiting assessment' and contacted study authors for clarification. We presented an adapted PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) flow diagram showing the process of study selection (Liberati 2009).
Data extraction and management
For trials that fulfilled inclusion criteria, two review authors (two of RJ, LA, EL, JC, WR) independently abstracted key participant and intervention characteristics and reported data on efficacy outcomes and adverse events using standard data extraction templates as supplied by the CMED group, with any disagreements resolved by discussion, or, if required, by consultation with a third review author (KR) (for details see Table 5; Appendix 2; Appendix 3; Appendix 4; Appendix 5; Appendix 6; Appendix 7; Appendix 8; Appendix 9).
1. Overview of study populations.
| Intervention(s) and comparator(s) | Sample sizea | Screened/eligible [N] | Randomised [N] | ITT [N] | Analysed [N] | Finishing trial [N] | Randomised finishing trial [%] | Follow‐up (extended follow‐up)a | |
| (20) Resnicow 2015 | I1: parent‐only PCP motivational interviewing | The study was powered to detect a 3‐point difference in BMI percentile between any pair of study groups at 2‐year follow‐up, with an assumed SD for BMI percentile between 4 and 6: power of 0.80 and 2‐tailed a of 0.05. Sample size was inflated to account for practice‐level clustering, assuming an intraclass correlation between 0.01 and 0.05. On this basis and a projected 25‐30% attrition at 2‐year follow‐up, 10‐12 practices per arm (30‐36 total) and a mean of 15‐20 children per practice at baseline were required | ‐ | 16 practices 212 participants |
145 | 145 | 145 | 68 | 2 years (2 years) |
| I2: parent‐only PCP + dietician motivational interviewing | 15 practices 235 participants |
154 | 154 | 154 | 66 | ||||
| C: usual care | 11 practices 198 participants |
158 | 158 | 158 | 80 | ||||
| total: | 645 | 457 | 457 | 457 | 71 | ||||
| (19) Mazzeo 2014 | I: parent NOURISH | ‐ | 235 | 48 | ‐ | 46 | 10 | 21 | 12 weeks post 12‐week intervention (24 weeks) |
| C: parent control | 45 | ‐ | 45 | 16 | 36 | ||||
| total: | 93 | 91 | 26 | 28 | |||||
| (18) Van Grieken 2013 | I: parent‐only | Sample size was calculated taking into account the intracluster correlation coefficient (ρ = 0.1), the number of clusters (44), the expected prevalence of overweight children in the study population, the SD, expected effect (a difference in mean), and the power of the study (80%). With a participation of 50%, an expected prevalence of overweight children of 9% and a loss‐to‐follow‐up of 30%, at least 11,301 children (and their parents) should be invited by the YHC teams to participate in the study to have a final sample of about 356 overweight children (178 in both the intervention and control group). Assuming a SD of BMI to be 1.0 kg/m2, a difference in mean BMI of 0.35 kg/m2 between the children in the intervention group and the children in the control group can be established under the assumptions mentioned above | 22 clusters 7004 participants |
349 | ‐ | 21 clusters 277 participants |
277 | 79 | 2 years post up to 12‐month intervention |
| C: usual care | 22 clusters 7004 participants |
288 | ‐ | 21 clusters 230 participants |
230 | 80 | |||
| total | 637 | ‐ | 42 (507) | 507 | 80 | ||||
| (17) Small 2013 | I: parent‐only | ‐ | ‐ | 34 | 33 | 33 | 33 | 97 | 24 weeks post 16‐week intervention (41 weeks) |
| C: parent control | 33 | 27 | 27 | 27 | 82 | ||||
| total: | 67 | 60 | 60 | 60 | 90 | ||||
| (16) Esfarjani 2013 | I: parent‐only | ‐ | 550/156 | 70 | ‐ | 55 | 58 | 83 | Intervention 6 months (not reported) |
| C: parent control | 86 | ‐ | 52 | 59 | 69 | ||||
| total: | 156 | ‐ | 107 | 117 | 75 | ||||
| (15) Moens 2012 | I: parent‐only | ‐ | 80/75 | 31 | ‐ | ‐ | 31 | 100 | Immediately following 6‐month intervention |
| C: waiting list control | 19 | ‐ | ‐ | 15 | 79 | ||||
| total: | 50 | ‐ | ‐ | 46 | 92 | ||||
| (14) Raynor 2012a | I1: parent‐only | Sample size calculations presumed 2‐sided hypothesis testing at 6‐month assessment, with type 1 error rate = 0.05. To reject with 80% power the null hypothesis of no pre‐ to post‐treatment difference between intervention conditions vs. the alternative that the pre‐ to post‐treatment difference was 0.6 or greater (effect size), 24 participants per group were needed | 549 | 33 | 33 | 33 | 29 | 88 | 24 weeks post 24‐week intervention (reported as '12 months') |
| I2: parent ‐ diet decrease | 33 | 33 | 33 | 29 | 88 | ||||
| I3: parent ‐ diet increase | 35 | 35 | 35 | 32 | 91 | ||||
| total: | 101 | 101 | 101 | 90 | 89 | ||||
| (13) Raynor 2012b | I1: parent‐only | Sample size calculations presumed 2‐sided hypothesis testing at 6‐month assessment, with type 1 error rate = 0.05. To reject with 80% power the null hypothesis of no pre‐ to post‐treatment difference between intervention conditions vs. the alternative that the pre‐ to post‐treatment difference is 0.6 or greater (effect size), 24 participants per group were needed | 549 | 29 | 29 | 29 | 26 | 90 | 24 weeks post 24‐week intervention (reported as '12 months') |
| I2: parent ‐ diet and activity traditional | 26 | 26 | 26 | 24 | 92 | ||||
| I3: parent ‐ diet and activity substitute | 26 | 26 | 26 | 24 | 92 | ||||
| total: | 81 | 81 | 81 | 74 | 91 | ||||
| (12) Margarey 2011 | I: parent healthy lifestyle | Sample size calculation was based on a reduction in BMI z score of 0.26 (SD 0.49) over 12 months (power 80%, alpha = 0.05, and drop‐out rate of 30%). This represents a 50% reduction in weight velocity over 12 months and no change in height velocity. We sought 42 children per group per site (168 children) | 398 | 85 | 85 | 85 | 52 | 61 | 80 weeks post 24‐week intervention (104 weeks) |
| C: healthy lifestyle | 84 | 84 | 84 | 54 | 64 | ||||
| total: | 169 | 169 | 169 | 106 | 63 | ||||
| (11) Jansen 2011 | I: parent CBT | ‐ | 161 | 59 | ‐ | 54 | 54 | 92 | 12 weeks post 12‐week intervention (24 weeks) |
| C: waiting list control | 39 | ‐ | 34 | 34 | 87 | ||||
| total: | 98 | 88 | 88 | 90 | |||||
| (10) Collins 2011 | I: parent‐only ‐ diet | Power: 80% chance of detecting significance (2‐sided 5% level), with a 0.26 BMI z score difference from baseline to 12 months as the initial end point, with an anticipated loss to follow‐up of 20% | 505/319 | 63 | ‐ | 42 | 22 | 35 | 80 weeks post 24‐week intervention (104 weeks) |
| C1: parent‐child (physical activity) | 73 | ‐ | 63 | 35 | 48 | ||||
| C2: parent‐child (physical activity + diet) | 70 | ‐ | 60 | 36 | 51 | ||||
| total: | 206 | ‐ | 165 | 93 | 45 | ||||
| (9) Boutelle 2011 | I: parent‐only | Sample size was determined by pragmatic factors, including budget and investigator time commitments. No interim analyses were done. The hypotheses tested related to non‐inferiority of the parent treatment to the parent‐child treatment on child and parent weight loss and child daily caloric intake and physical activity. The bound for non‐inferiority hypotheses related to BMI percentile was set to 1. This is the maximum value the parent‐child group could do better than parent‐only, below which non‐inferiority would be concluded. This bound could correspond to an mean‐aged child in this sample having a BMI of 26 in the parent‐child group and 28.5 in the parent‐only group at post‐treatment/follow‐up, assuming equivalence at baseline. For a non‐inferiority bound for child BMI, which was selected post hoc, we considered choosing a BMI that would correspond to the BMI percentile non‐inferiority bound (BMI = 2.5), but instead chose a more rigorous value of BMI = 1 | 157 | 40 | ‐ | 24 | 24 | 60 | 24 weeks post 20‐week intervention (week 44) |
| C: parent‐child | 40 | ‐ | 28 | 28 | 70 | ||||
| total: | 80 | 52 | 52 | 65 | |||||
| (8) West 2010 | I: parent‐only | ‐ | 205 | 52 | 52 | 52 | 34 | 65 | 40 weeks post 12‐week intervention (52 weeks) |
| C: waiting list control | 49 | 49 | 49 | 46 | 94 | ||||
| total: | 101 | 101 | 101 | 80 | 79 | ||||
| (7) Resnick 2009 | I: educational material + personal encounters | ‐ | 84/46 | 22 | ‐ | 18 | 18 | 82 | Unclear (41 weeks between start and last mail out) |
| C: educational material | 24 | ‐ | 24 | 24 | 100 | ||||
| total: | 46 | 42 | 42 | 91 | |||||
| (6) Estabrooks 2009 | I1: parent group + IVR | Sample size calculations were completed, varying the detectable effect sizes from small to medium with a power of 0.8. The result was a need for 42 participants per intervention to detect a medium effect and 64 participants to detect a small effect | 1487/656 | 85 | ‐ | 63 | 63 | 74 | 28‐40 weeks post 12‐ to 24‐week intervention (52 weeks) |
| I2: parent group | 85 | ‐ | 56 | 56 | 66 | ||||
| C: parent workbook | 50 | ‐ | 36 | 36 | 72 | ||||
| total: | 220 | 155 | 155 | 70 | |||||
| (5) Munsch 2008 | I: mother‐only CBT | Trial authors did not reach the necessary sample size of 68 families with obese children within the given time span (the target sample size of 68 was based on a repeated‐measures analysis with alpha = 0.05, 1 ‐ beta = 0.8, and a medium effect size for the linear term of the interaction between treatment and time, assuming a drop‐out rate of 20% | 181/60 | 25 | ‐ | 7 | 7 | 28 | 24 weeks post 10‐week intervention (34 weeks) |
| C: mother‐child CBT | 31 | ‐ | 20 | 20 | 65 | ||||
| total: | 56 | 27 | 27 | 48 | |||||
| (4) Janicke 2008 | I: parent‐only | Post hoc power analyses were used to determine the detectable change in BMI z score from 0 to 10 months for the family based and parent‐only interventions relative to the waiting list control condition. Effect sizes (standardised BMI index) detectable with 80% power and 2‐sided level 0.05 tests were used. Standard deviations and sample sizes were set equal to their observed values. For comparing the family‐based and waiting list control conditions, trial authors reported 80% power to detect a shift from 0.022 to ‐0.145. For comparing the parent‐only and waiting list control conditions, trial authors reported 80% power to detect a shift from 0.022 to ‐0.135 | 111 | 34 | ‐ | 26 | 26 | 76 | 14 weeks post 16‐week intervention (40 weeks) |
| C1: parent‐child | 33 | ‐ | 24 | 24 | 73 | ||||
| C2: waiting list control | 26 | ‐ | 21 | 21 | 81 | ||||
| total: | 93 | 71 | 71 | 76 | |||||
| (3) Golley 2007 | I: parent intervention + lifestyle education | Sample size calculation was based on a fall in BMI z score reflecting a weight gain of only 50% of that expected over 12 months with normal growth. A sample size of 28 per group was estimated to have 80% power to detect a 12‐month fall in mean BMI z score from a baseline of 0.26 (SD 0.49), assuming no change in the control group, at a 2‐sided significance level of 0.05. To account for a drop‐out rate of up to one‐third (commonly 20‐50% in child weight‐management studies), 42 children per study group were sought (126 children) | 262/115 | 38 | ‐ | 31 | 31 | 82 | 24 weeks post 24‐week intervention (48 weeks) |
| C1: parent intervention | 37 | ‐ | 29 | 29 | 78 | ||||
| C2: waiting list control | 36 | ‐ | 31 | 31 | 86 | ||||
| total: | 111 | 101 | 101 | ‐ | |||||
| (2) Golan 2006 | I: parent‐only | The study was designed to detect differences of 10% weight loss with a power of 90% and a significance level of 0.05, given a drop‐out rate of 10% with a sample of 12 in each group | 102 | 14 | ‐ | 10 | 10 | 71 | 1 year post 26‐week intervention (18 months) |
| C: parent‐child | 18 | ‐ | 17 | 17 | 94 | ||||
| total: | 32 | 27 | 27 | 84 | |||||
| (1) Aragona 1975 | I1: parent‐only with reinforcement | ‐ | ‐ | 5 | ‐ | 4 | 4 | 80 | 12 weeks (51 weeks' follow‐up) |
| I2: parent‐only | 5 | ‐ | 3 | 3 | 60 | ||||
| C: waiting list control | 5 | ‐ | 5 | 2 | 40 | ||||
| total: | 15 | 12 | 9 | 60 | |||||
| Grand total | All interventions | ‐ | 1773c | 1276 | |||||
| All comparators | 1284c | 942 | |||||||
| All interventions and comparators | 3057c | 2218 | |||||||
aAccording to power calculation in trial publication or report bDuration of intervention or follow‐up (or both) under randomised conditions until end of trial cSome trials had more than one intervention/comparator group
"‐" denotes not reported
BMI: body mass index; C: comparator; CBT: cognitive behavioural therapy; I: intervention; ITT: intention‐to‐treat; IVR: interactive voice response; n: number of participants; NOURISH: nourishing our understanding of role modelling to improve support and health; PCP: primary care providers; SD: standard deviation; YHC: Youth Health Care
We provided information including trial identifier about potentially relevant ongoing trials in the Characteristics of ongoing studies table and in the 'Matrix of study endpoints (publications and trial documents)' where available. We tried to find the protocol of each included study in trial registers or publications of study designs, or both, and reported primary, secondary and other outcomes in comparison with data in publications in Appendix 5.
We emailed all authors of included trials to enquire whether they were willing to answer questions regarding their trials. Appendix 10 shows the results of this survey. Thereafter, we sought relevant missing information on the trial from the primary author(s) of the article, if required.
Dealing with duplicate and companion publications
In the event of duplicate publications, companion documents or multiple reports of a primary study, we tried to maximise yield of information by collating all available data and use the most complete data set aggregated across all known publications. In case of doubt, we gave priority to the publication reporting the longest follow‐up associated with our primary or secondary outcomes.
Assessment of risk of bias in included studies
Two review authors (two of RJ, LA, EL, JC) assessed the risk of bias of each included study independently. We resolved possible disagreements by consensus, or with consultation with a third review author (KR).
We used Cochrane's tool for assessing risk of bias (Higgins 2011a; Higgins 2011b), and evaluated the following criteria.
Random sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants and personnel (performance bias).
Blinding of outcome assessment (detection bias).
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Other potential sources of bias.
We judged the above risk of bias criteria as 'low risk', 'high risk' or 'unclear risk' and evaluated individual bias items as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We presented a 'Risk of bias' graph and a 'Risk of bias' summary. We assessed the impact of individual bias domains on study results at endpoint and study levels. In case of high risk of selection bias, we marked all endpoints investigated in the associated study as high risk.
We evaluated whether imbalances in baseline characteristics existed and how these were addressed (Egbewale 2014).
For performance bias (blinding of participants and personnel) and detection bias (blinding of outcome assessors), we evaluated the risk of bias separately for each outcome type (objective and subjective) (Hróbjartsson 2013).
We considered the implications of missing outcome data from individual participants per outcome such as high drop‐out rates (e.g. above 15%) or disparate attrition rates (e.g. difference of 10% or more between study arms).
We assessed outcome reporting bias by integrating the results of 'Examination of outcome reporting bias' (Appendix 6) and the 'Matrix of study endpoints (publications and trial documents)' (Appendix 5) (Kirkham 2010). This analysis formed the basis for the judgement of selective reporting (reporting bias).
We defined the following as self reported outcomes ('subjective outcomes').
Adverse events.
Health‐related quality of life and self esteem.
Parent‐child relationship or assessment of parenting.
Participants' views of the intervention.
We defined the following as investigator‐assessed outcomes ('objective outcomes').
Changes in BMI measures and body weight.
Measures of body fat distribution.
Adverse events.
All‐cause mortality.
Morbidity.
Behaviour change.
Socioeconomic effects.
Measures of treatment effect
We expressed dichotomous data as odds ratios (ORs) or risk ratios (RRs) with 95% confidence intervals (CIs). We expressed continuous data as mean differences (MD) with 95% CI. We planned to express time‐to‐event data as hazard ratios (HRs) with 95% CIs.
Unit of analysis issues
We took into account the level at which randomisation occurred, such as cross‐over trials, cluster‐randomised trials and multiple observations for the same outcome. For cluster‐randomised trials, we used the adjusted data reported in the original studies. Where studies had multiple control groups, we used data from the control group for each comparison by reducing the weight assigned to the control group by dividing the number of participants in the control group by the number of intervention groups.
Dealing with missing data
We obtained relevant missing data from authors, if possible, and evaluated important numerical data such as screened, eligible, randomised participants as well as intention‐to‐treat (ITT), as‐treated and per‐protocol (PP) populations where possible. We investigated attrition rates, for example drop‐outs, losses to follow‐up and withdrawals, and critically appraise issues of missing data and imputation methods (e.g. last‐observation‐carried‐forward (LOCF)).
Where standard deviations (SD) for outcomes were not reported, and we did not receive information from study authors, we calculated these following the methods presented in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). Where papers did not report results as change from baseline, we calculated this and for the SD differences followed the methods presented in the Cochrane Handbook for Systematic Reviews of Interventions for imputing these (Section 16.1.3.2 Imputing standard deviations for changes from baseline; Higgins 2011a), and assumed a correlation of 0.5 between baseline and follow‐up measures as suggested by Follman 1992.
Assessment of heterogeneity
In the event of substantial clinical or methodological heterogeneity, we did not report study results as meta‐analytically pooled effect estimates. We identified heterogeneity by visual inspection of the forest plots and by using a standard Chi2 test with a significance level of α = 0.1, in view of the low power of this test. We examined heterogeneity using the I2 statistic, which quantifies inconsistency across trials to assess the impact of heterogeneity on the meta‐analysis (Higgins 2002; Higgins 2003), where an I2 statistic of 75% or more indicates a considerable level of inconsistency (Higgins 2011a).
When we found heterogeneity, we attempted to determine potential reasons for it by examining individual study and subgroup characteristics.
Assessment of reporting biases
If we had included 10 trials or more for a given outcome, we would have used funnel plots to assess small‐study effects. Due to several explanations for funnel plot asymmetry, we would have interpreted results carefully (Sterne 2011).
Data synthesis
Unless there was good evidence for homogeneous effects across trials, we primarily summarised data by means of a random‐effects model (Wood 2008). We had planned to interpret random‐effects meta‐analyses with due consideration of the whole distribution of effects, ideally by presenting a prediction interval; however, there were relatively few trials included in each category, of low methodological quality and so theses analyses were not conducted (Higgins 2009). A prediction interval specifies a predicted range for the true treatment effect in an individual study (Riley 2011). We performed statistical analyses according to the statistical guidelines referenced in the latest version of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
Quality of evidence
We present the overall quality of the evidence for each outcome according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, which takes into account issues not only related to internal validity (risk of bias, inconsistency, imprecision, publication bias) but also to external validity such as directness of results. Two review authors (EL, KR) rated the quality for each outcome. We presented a summaries of the evidence in a 'Summary of findings' tables, which provide key information about the best estimate of the magnitude of the effect, in relative terms and absolute differences for each relevant comparison of alternative management strategies, numbers of participants and trials addressing each important outcome, and the rating of the overall confidence in effect estimates for each outcome. We created the 'Summary of findings' tables based on the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We presented results on the outcomes as described in Types of outcome measures.
In addition, we established an appendix 'Checklist to aid consistency and reproducibility of GRADE assessments' (Meader 2014) to help with standardisation of 'Summary of findings' tables (Appendix 9).
Subgroup analysis and investigation of heterogeneity
We expected the following characteristics to introduce clinical heterogeneity, and aimed to carry out subgroup analyses with investigation of interactions where data permitted.
Differences in BMI at baseline.
Length of follow‐up.
The impact of comparator/control: whether concomitant therapy or no treatment (true control).
The setting in which the intervention was conducted.
Sensitivity analysis
We planned to perform sensitivity analyses to explore the influence of the following factors on effect size.
Restricting the analysis to published trials.
Restricting the analysis taking into account risk of bias, as specified in the 'Assessment of risk of bias in included studies' section.
Restricting the analysis to very long or large trials to establish how much they dominated the results.
Restricting the analysis to trials using the following filters: diagnostic criteria, language of publication, source of funding (industry versus other), country.
We tested the robustness of the results by repeating the analysis using different statistical models (fixed‐effect and random‐effects models).
Results
Description of studies
For a detailed description of trials, see the Characteristics of included studies, Characteristics of excluded studies and Characteristics of ongoing studies tables.
Results of the search
The searches generated 13,759 hits after duplicates were removed. Screening of titles and abstracts identified 137 papers to go forward for formal inclusion and exclusion. Twenty completed RCTs fulfilled the inclusion criteria and were included in the review. For a detailed description of the included trials, see the Characteristics of included studies table. The search identified 10 ongoing trials, which are reported in the Characteristics of ongoing studies table. The flow of trials through the review is presented in Figure 1.
1.
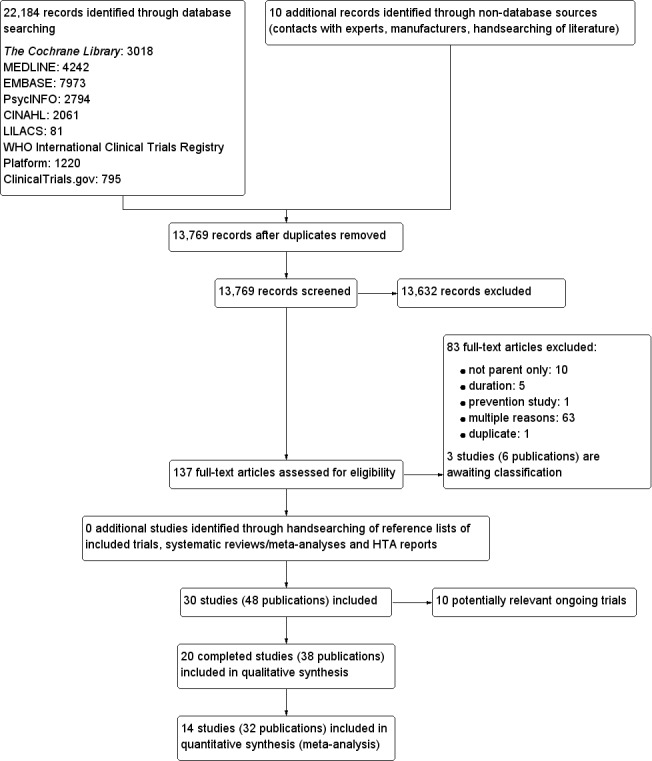
Study flow diagram.
Included studies
A detailed description of the characteristics of included trials is presented elsewhere (see Characteristics of included studies table and Appendix 2; Appendix 3; Appendix 4; Appendix 5; Appendix 6; Appendix 7; Appendix 8; Appendix 9). The following is a succinct overview.
Source of data
The majority of data presented in the review was obtained from published literature, including supplementary published data where available. For five trials, trial authors provided data (Appendix 10).
Study details
We included 20 RCTs in 19 main publications (Aragona 1975; Boutelle 2011; Collins 2011; Esfarjani 2013; Estabrooks 2009; Golan 2006; Golley 2007; Janicke 2008; Jansen 2011; Magarey 2011; Mazzeo 2014; Munsch 2008; Raynor 2012a; Raynor 2012b; Resnick 2009; Resnicow 2015; Small 2013; van Grieken 2013; West 2010). Eighteen RCTs were parallel comparisons with individual randomisation. In most trials, the unit of randomisation was the family (parent and child); however, study authors analysed the children and parents for respective outcomes separately. Two RCTs were cluster RCTs, where the Primary Care Provider (PCP) (Resnicow 2015) or Youth Health Care (YHC) (van Grieken 2013) team were the unit of randomisation. Eighteen RCTs were superiority trials, one had a non‐inferiority study design (Boutelle 2011), and one an equivalence study design (Munsch 2008). Eight RCTs had three comparisons (Aragona 1975; Collins 2011; Estabrooks 2009; Golley 2007; Janicke 2008; Raynor 2012a; Raynor 2012b; Resnicow 2015); the remaining trials had two comparison groups.
Ten trials were undertaken in the USA (Aragona 1975; Boutelle 2011; Estabrooks 2009; Janicke 2008; Mazzeo 2014; Raynor 2012a; Raynor 2012b; Resnick 2009; Resnicow 2015; Small 2013); four in Australia (Collins 2011; Golley 2007; Magarey 2011; West 2010), and two in the Netherlands (Jansen 2011; van Grieken 2013). There was one trial each from Israel (Golan 2006), Switzerland (Munsch 2008), Iran (Esfarjani 2013), and Belgium (Moens 2012). Five trials were single‐centre trials (Aragona 1975; Boutelle 2011; Esfarjani 2013; Golan 2006; Moens 2012); the remaining trials were either multi‐centre trials (with two centres: Golley 2007; Munsch 2008; Resnick 2009; three centres: Jansen 2011; Magarey 2011; and six centres: West 2010), or the numbers of centres were not reported (Collins 2011; Estabrooks 2009; Janicke 2008; Mazzeo 2014; Raynor 2012a; Raynor 2012b; Small 2013). The cluster RCT by van Grieken 2013 was undertaken in nine study centres, across 44 healthcare teams. The cluster RCT by Resnicow 2015 was undertaken in 42 primary care practices.
Overview of study populations
All trials included parents of overweight or obese children. The diagnostic criteria differed between trials, in 10 trials this was based on the BMI 85th percentile (Boutelle 2011; Estabrooks 2009; Golan 2006; Janicke 2008; Mazzeo 2014; Munsch 2008; Raynor 2012a; Raynor 2012b; Resnick 2009; Resnicow 2015), and in one trial the BMI 95th percentile (Esfarjani 2013). In other trials, this was based on the parent or physician describing their child as overweight (Aragona 1975; Moens 2012; Small 2013; West 2010); the IOTF definition (Golley 2007; Magarey 2011), or stated as international cut‐off points that were not specified (van Grieken 2013); a specified BMI cut‐off (Collins 2011); or the proportion of BMI above expected BMI (at least 30% greater in Jansen 2011).
All trials included parents of children aged between 4 and 13 years, the majority of which did not include children above 11 years of age. The mean ages of participants were reported in 16 trials. In six trials, the mean ages were between 5 and 7 years (Esfarjani 2013; Raynor 2012a; Raynor 2012b; Resnicow 2015; Small 2013; van Grieken 2013); in seven trials, the mean ages were between 8 and 9 years (Aragona 1975; Collins 2011; Golan 2006; Magarey 2011; Moens 2012; West 2010); and in four trials, the mean ages of the children was between 10 and 11 years (Boutelle 2011; Estabrooks 2009; Janicke 2008; Munsch 2008). The proportion of girls in the trials typically ranged from 40% to 70% where reported (four trials did not report this: Esfarjani 2013; Janicke 2008; Mazzeo 2014; Resnick 2009), although was 100% in one study (Aragona 1975). Only eight trials reported ethnicity of the children and in all trials there was a high proportion of children categorised as white (between 60% and 100% across all trials: Estabrooks 2009; Janicke 2008; Moens 2012; Raynor 2012a; Raynor 2012b; Resnicow 2015; Small 2013; West 2010). Only eight trials reported socioeconomic indices of the parents (Boutelle 2011; Esfarjani 2013; Janicke 2008; Moens 2012; Resnicow 2015; Small 2013; van Grieken 2013; West 2010). Each used a different indicator of socioeconomic status (see Appendix 4).
The number of participants included in the 19 trials ranged between 15 and 645. Five trials had fewer than 30 participants per study arm (Aragona 1975; Moens 2012; Munsch 2008; Raynor 2012b; Resnick 2009), whereas four trials had greater than 60 participants per study arm (Collins 2011; Esfarjani 2013; Estabrooks 2009; Magarey 2011). In the cluster RCT by van Grieken 2013, there were 22 clusters in each arm (total 637 participants), in the Resnicow 2015 cluster RCT, there were 42 clusters (total 645 participants).
Seven trials reported the BMI z score, which ranged from 2.0 to 2.8 at baseline (Boutelle 2011; Collins 2011; Estabrooks 2009; Golley 2007; Janicke 2008; Magarey 2011; West 2010). Five trials reported the BMI percentile, which ranged from 92% to 98.5% at baseline (Jansen 2011; Mazzeo 2014; Resnick 2009; Resnicow 2015; Small 2013). Five trials reported BMI (Esfarjani 2013; Mazzeo 2014; Munsch 2008; Raynor 2012a; Raynor 2012b), and could be calculated from individual participant data in one other study (Aragona 1975). The BMI in these trials ranged from 22 to 33.6 at baseline. Eleven trials reported parental BMI. In eight trials, the mean parental BMI ranged between 26 and 36; with four trials including parents with BMIs between 26 and 30 (Jansen 2011; Munsch 2008; Resnick 2009; Resnicow 2015), and four trials including parents with BMIs above 30 (Boutelle 2011; Janicke 2008; Mazzeo 2014; Small 2013). One study reported the proportions of parents in three categories (healthy, overweight, obese); these were approximately 37% (healthy), 23% (overweight) and 40% (obese) (West 2010). Another study reported the proportions of 'normal' (about 56%) and 'overweight' (about 44%) (van Grieken 2013). One study reported the weight of the parents (for mothers and fathers for each group respectively) (Golan 2006).
For details of baseline characteristics of participants in the included trials, see Appendix 4 and Appendix 5. There were no substantial differences in baseline characteristics between the intervention and comparator groups in the included trials.
Interventions
Seventeen trials reported the settings for the interventions. In four trials, the intervention was in an outpatient setting (Collins 2011; Estabrooks 2009; Golley 2007; Magarey 2011), and in four trials it was a community setting (Janicke 2008; Mazzeo 2014; Resnick 2009; van Grieken 2013). Two trials were undertaken in a university setting (Boutelle 2011; Moens 2012); two in a primary care setting (Resnicow 2015; Small 2013), and five trials win a mixture of settings including outpatient, university, primary care or a combination of these settings (Jansen 2011; Munsch 2008; Raynor 2012a; Raynor 2012b; West 2010).
The interventions in the included trials predominantly focused on nutritional, physical activity and behavioural components; see Characteristics of included studies table and Appendix 2 for specific details for each included study.
Five trials compared a parent‐only intervention to a parent‐child intervention (two also had a third comparison of a waiting list control, Janicke 2008 or a second parent‐child intervention, Collins 2011) (Boutelle 2011; Collins 2011; Golan 2006; Janicke 2008; Munsch 2008). In one study, the duration of the intervention was 10 weeks (Munsch 2008); and four trials had interventions of 16 to 24 weeks' duration (Boutelle 2011; Collins 2011; Golan 2006; Janicke 2008). Session lengths ranged from one to two hours in all five trials. All trials followed participants beyond the timing of the end of the intervention. This was 14 weeks' post intervention in one study (Janicke 2008), 24 to 26 weeks' post intervention in two trials (Boutelle 2011; Munsch 2008), one year post intervention in one trial (Golan 2006), and 80 weeks' post intervention in one study (Collins 2011).
Six trials compared a parent‐only intervention to a waiting list control (Aragona 1975; Golley 2007; Janicke 2008; Jansen 2011; Moens 2012; West 2010) and two of these trials had two different parent‐only interventions (Aragona 1975; Golley 2007). The duration of the intervention ranged from 12 to 24 weeks in these trials and sessions ranged between 90 minutes and two hours (one study did not provide details, Aragona 1975). In two trials, there were additional sessions via telephone contact between interventionists and the parents, which were approximately 20 minutes long (Golley 2007; West 2010). All but one study followed participants up beyond the timing of the end of the intervention (Moens 2012). This was 12 to 14 weeks post intervention in one trial (Jansen 2011), 24 weeks post intervention in two trials (Golley 2007; Janicke 2008), 39 weeks post intervention in one trial (Aragona 1975) and 40 weeks post intervention in one trial West 2010).
Seven trials compared a parent‐only intervention with a minimal contact parenting advice control (mailed information or a workbook or minimal sessions) (Esfarjani 2013; Estabrooks 2009; Mazzeo 2014; Resnick 2009; Resnicow 2015; Small 2013; van Grieken 2013); two trials had two different parent‐only interventions (Estabrooks 2009; Resnicow 2015). The duration of the interventions ranged from 12 to 26 weeks in five trials (in the Resnick 2009 study the interventions ranged from 30 to 41 weeks but the study reports a mean of 18 weeks). In one study, the duration of the intervention differed for each participants, but was up to 12 months (van Grieken 2013). In one trial, the intervention continued to 24 months (Resnicow 2015). In three trials, the number of sessions provided to parents was lower than reported in the other parent‐only interventions, being three or four sessions (Resnick 2009; Small 2013; van Grieken 2013). In two trials, the intervention was delivered across 12 sessions (Esfarjani 2013; Mazzeo 2014); in the study by Estabrooks 2009, there were two parent‐only treatment arms, which were either 12 or 24 weeks in duration and in the study by Resnicow 2015, there were also two parent‐only treatment arms, which were either four or 10 sessions. Six trials reported the duration of follow‐up in relation to the completion of the intervention. In the study by Mazzeo 2014, this was 12 weeks post intervention completion, in Small 2013, this was 24 weeks post completion of the intervention, in Estabrooks 2009, this was between 28 and 40 weeks post completion of the intervention and in Aragona 1975, this was 39 weeks post completion of the intervention. In the van Grieken 2013 RCT, follow‐up was 24 months after baseline (the duration of the intervention was "up to 12 months") and in the Resnicow 2015 trial, follow‐up was at completion of the intervention at 24 months.
Seven trials compared different types of parent‐only interventions (Aragona 1975; Estabrooks 2009; Golley 2007; Magarey 2011; Raynor 2012a; Raynor 2012b; Resnicow 2015), two also had a parent‐only versus waiting list control comparison (Aragona 1975; Golley 2007), and two trials also had a parent‐only versus minimal contact intervention (Estabrooks 2009; Resnicow 2015). In the first, by Estabrooks 2009, there were three treatment arms. In one treatment group, the parents received a self help workbook, attended two group sessions and these were followed up with 10 interactive voice response (IVR) counselling sessions over a 10‐week period. In the second arm, the parents received the work book and attended the same two group sessions but did not have the IVR follow‐up sessions. In the third group, parents received the workbook only. In the study by Resnicow 2015, participants either received motivational interviewing over four sessions in the primary care practice, or received the four motivational interviewing sessions and six sessions with a dietician (the third arm received usual care information). In the study by Magarey 2011, parents were randomised into two arms, one received a four‐session parenting programme that was followed by eight group sessions about healthy lifestyles. The second group received the eight sessions about healthy lifestyles only. Both the trials by Estabrooks 2009 and Magarey 2011 followed participants up beyond the timing of the end of the intervention. In the study by Estabrooks 2009, this was between 28 and 40 weeks post completion of the intervention and in the study by Magarey 2011, this was 80 weeks post completion of the intervention. In the study by Resnicow 2015, follow‐up was at the point of completing the intervention (24 weeks) although in the second 12‐month period of the intervention there were fewer sessions held.
Two trials in one publication also compared different parent‐only interventions (Raynor 2012a; Raynor 2012b). There were many shared attributes of the two trials. The first trial compared a parent‐only intervention that focused on growth monitoring to a parent intervention that focused on decreasing sugary foods in the diet and a parent intervention that focused on increasing healthy food consumption (Raynor 2012a). Parents randomised to the two comparison groups also received the parent‐only intervention (growth monitoring). The second study compared the parent‐only intervention to a parent intervention that focused on decreasing sugary foods and increasing physical activity, and to a parent intervention that focused on increasing low‐fat milk intake and decreasing television watching (Raynor 2012b). Parents randomised to the two comparison groups also received the parent‐only 'growth monitoring' intervention. The duration of the interventions in these two trials was six months, and there were eight sessions of 45 minutes' duration. These trials followed participants up 24 weeks after the timing of the end of the intervention. Golley 2007 compared parenting skills training with the addition of intensive lifestyle education with the parenting skills training without the additional intensive lifestyle education. The duration of the interventions are as described above. Aragona 1975 compared a parenting response‐cost and training in reinforcement skills with the response‐cost training only. Parents were required to enter a contract with the interventionists and pay a deposit relating to a goal of weight loss in their child, which could be returned for attendance at the sessions and when the goal was achieved. The duration of the interventions is as described above.
Six trials reported compliance rates with the interventions (Estabrooks 2009; Golan 2006; Golley 2007; Mazzeo 2014; Raynor 2012a; Raynor 2012b). One study reported that attendance in both arms of their trial was above 80% (Golan 2006). In two trials, the trial authors reported that attendance at growth monitoring appointments did not differ among the groups (Raynor 2012a; Raynor 2012b). In Raynor 2012a, compliance with attendance and turning in monitoring dairies was 73%, in Raynor 2012b this was 64%. In the study by Mazzeo 2014, the numbers attending 50% or more of sessions were reported for the parent‐only intervention (22/43). Mean session attendance across groups was 53%. In the control group, 35/41 parents attended the single session. Golley 2007 reported the number of participants attending sessions in the two intervention arms. There were 11 sessions in the parenting‐skill training arm and 19 parents attended at least eight sessions; 13 attended 2 to 7 sessions and 5 attended 1 or 0 sessions. In the parenting‐skills training with intensive lifestyle education arm, there were 18 sessions and the study reported that 18 parents attended at least 13 sessions; 18 attended 2 to 12 sessions; and 2 attended 1 or sessions. Estabrooks 2009 reported the number of participants taking part in the IVR intervention where 20 participants took part in 0 to 5 calls while 38 took part in 6 to 10 calls.
Outcomes
All trials reported weight, BMI, or both, as an outcome measure. Other outcomes reported in the trials differed with few similarities across the included trials in the choice of outcomes reported (see Appendix 5). Where reported, measures of BMI were reported differently across the trials. Where a study reported more than one measure of BMI, we took the BMI z score as the preferred measure in the data synthesis.
Excluded studies
We excluded 83 of 137 full‐text articles after evaluation of the full publication.
The main reasons for exclusion were the interventions also included the children (not parent‐only) and the duration of the study was less than six months. Many trials had multiple reasons for exclusion (for further details see Characteristics of excluded studies table, which lists the 16 trials that most closely missed the inclusion criteria).
Risk of bias in included studies
For details on risk of bias of included trials, see Characteristics of included studies table. For an overview of review authors' judgements about each risk of bias item for individual trials and across all trials, see Figure 2 and Figure 3.
2.
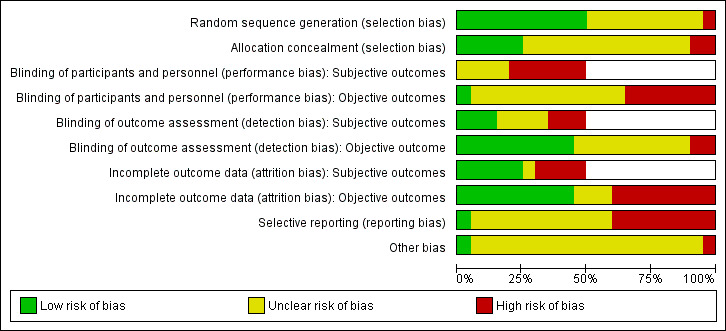
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies (blank cells indicate that the particular outcome was not investigated in some studies).
3.
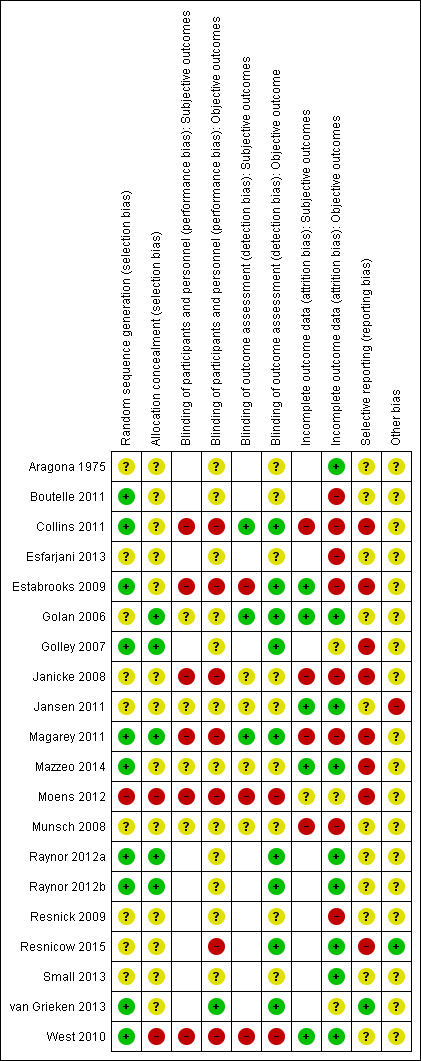
Risk of bias summary: review authors' judgements about each risk of bias item for each included study (blank cells indicate that the study did not report that particular outcome).
Trial quality was generally low. Many trials did not report adequate information to assess the risk of bias and we assessed 14 trials at high risk of bias on at least one domain (Boutelle 2011; Collins 2011; Esfarjani 2013; Estabrooks 2009; Golley 2007; Janicke 2008; Jansen 2011; Magarey 2011; Mazzeo 2014; Moens 2012; Munsch 2008; Resnick 2009; Resnicow 2015; West 2010). We assessed six trials at high risk of bias on three or more domains (Collins 2011; Estabrooks 2009; Janicke 2008; Magarey 2011; Moens 2012; West 2010).
Allocation
Only 10 of 19 trials reported an adequate method of randomisation (Boutelle 2011; Collins 2011; Estabrooks 2009; Golley 2007; Magarey 2011; Mazzeo 2014; Raynor 2012a; Raynor 2012b; van Grieken 2013; West 2010). Five trials reported an adequate concealment of allocation (Golan 2006; Golley 2007; Magarey 2011; Raynor 2012a; Raynor 2012b).
Blinding
The potential for performance bias was unknown in most trials. Six trials were at high risk of performance bias for objective or subjective (or both) outcomes as appropriate to their study outcomes (Collins 2011; Estabrooks 2009; Magarey 2011; Moens 2012; Resnicow 2015; West 2010). Blinding of outcome assessors was adequate for objective outcomes in nine trials (Collins 2011; Estabrooks 2009; Golan 2006; Golley 2007; Magarey 2011; Raynor 2012a; Raynor 2012b; Resnicow 2015; van Grieken 2013). In three trials, the risk of detection bias was high for subjective outcomes (Estabrooks 2009; Moens 2012; West 2010).
Incomplete outcome data
Many trials reported high levels of drop‐out or loss to follow‐up (highest drop‐out rates ranged between 40% and 79%) and only nine trials reported adequate means to address these in the analysis (Aragona 1975; Golan 2006; Jansen 2011; Mazzeo 2014; Raynor 2012a; Raynor 2012b; Resnicow 2015; Small 2013; West 2010). Eight trials were at high risk of attrition bias on objective outcomes (Boutelle 2011; Collins 2011; Esfarjani 2013; Estabrooks 2009; Janicke 2008; Magarey 2011; Munsch 2008; Resnick 2009), and four trials on subjective outcome measures (Collins 2011; Janicke 2008; Magarey 2011; Munsch 2008).
Selective reporting
One trial was at low risk of selective reporting bias (van Grieken 2013), whereas eight trials were at high risk of selective reporting bias (Collins 2011; Estabrooks 2009; Golley 2007; Janicke 2008; Magarey 2011; Mazzeo 2014; Moens 2012; Resnicow 2015). All other trials were at unclear risk of selective reporting bias.
Other potential sources of bias
One study by Jansen 2011 was at high risk of bias because nine participant families who had originally been randomised to the waiting list control were included in the analysis for the parent‐only intervention. There were no other potential sources of bias identified by review authors and all of the remaining trials were at unclear risk of bias on this factor.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
The included trials had different durations of interventions and follow‐up. To assess the effects of the interventions we considered outcomes from the longest period of follow‐up in each study. In addition, we also reported outcomes from any post intervention follow‐up period (if it differed from the longest period of follow‐up). This allowed an assessment of the initial response to the intervention and any maintenance of that response on outcomes. If a study reported outcomes at any interim time points, we extracted these data as per the review protocol.
For 10 of 19 trials, we had to calculate SD as described (Dealing with missing data) (Boutelle 2011; Collins 2011; Esfarjani 2013; Estabrooks 2009; Jansen 2011; Magarey 2011; Mazzeo 2014; Resnick 2009; Small 2013; West 2010). Furthermore, one trial author provided SD data on two trials (Raynor 2012a; Raynor 2012b), after being contacted (Appendix 10).
Parent‐only interventions versus parent‐child interventions
Five trials reported seven comparisons of a parent‐only intervention and a parent‐child intervention (Boutelle 2011; Collins 2011; Golan 2006; Janicke 2008; Munsch 2008). To allow consideration of the effects of the interventions, we considered outcomes here from the longest period of follow‐up and any post intervention follow‐up. The period for the post intervention follow‐up in these trials ranged from 10 weeks to 12 months and the period for the longest point of follow‐up ranged from 8 to 24 months. Losses to follow‐up ranged from 6% to 50% at the post intervention follow‐up and 18% to 72% at the longest period of follow‐up (see Table 5). One study reported the participants completing the study but it was unclear what numbers of participants were included in the post intervention follow‐up (Golan 2006). In the trials by Golan 2006, Collins 2011, and Munsch 2008, there was a differential rate of losses to follow‐up between groups. All of these factors need to be considered when interpreting the results of the trials.
Primary outcomes
Changes in body mass index and body weight
Five trials reported BMI variables at the end of the intervention (Boutelle 2011 at five months; Collins 2011 and Golan 2006 at six months; Janicke 2008 at four months; Munsch 2008 at 10 weeks). All trials reported the BMI z score. Three trials (four comparisons) reported data that could be analysed in a meta‐analysis; Golan 2006 and Munsch 2008 did not report SDs or information that could be used to estimate SDs.
A pooled summary estimate of the change in BMI z score is shown in Analysis 1.1, the MD was ‐0.06 ((95% CI ‐0.13 to 0.02); P = 0.14; 277 participants; 3 trials with 4 treatment groups; low quality evidence). There was moderate heterogeneity (I2 = 37%), and similar results occurred with a fixed‐effect meta‐analysis. All included trials had a high risk of attrition bias. SDs were imputed for one trial (Boutelle 2011).
1.1. Analysis.
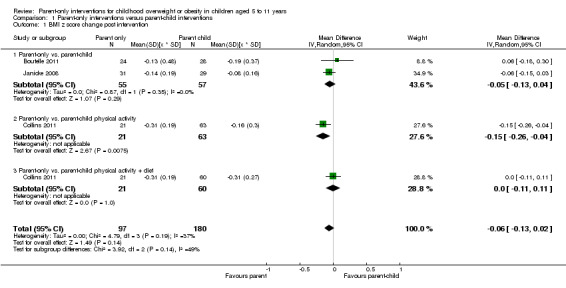
Comparison 1 Parent‐only interventions versus parent‐child interventions, Outcome 1 BMI z score change post intervention.
Of the two trials that did not report measure of variance, the mean change in BMI z score was reduced in both groups; ‐0.4 in the parent‐only group and ‐0.1 in the parent‐child group in the study by Golan 2006, and ‐0.16 in the parent‐only group and ‐0.08 in the parent‐child group in the study by Munsch 2008. All trials had either high rates of non‐completion across groups or differential non‐completion rates between study groups and, therefore, these results need to be interpreted with caution. In the study by Collins 2011, the BMI was also reported, there were no significant differences in change from baseline between groups.
Five trials (six comparisons) reported BMI variables beyond the end of the intervention (Boutelle 2011, Janicke 2008, and Munsch 2008 after six months; Collins 2011 after 18 months; Golan 2006 after 12 months). In all five trials, this was the BMI z score. Two trials did not report SDs or information that could be used to estimate SDs and, therefore, these were not included in the pooled summary estimate (Golan 2006; Munsch 2008). Three trials (four comparisons) therefore provided data that could be analysed in a meta‐analysis (Boutelle 2011; Collins 2011; Janicke 2008) (see Analysis 1.2). There was no substantial difference on BMI z score change between those in the parent‐only interventions and those in the parent‐child interventions ((MD ‐0.04 (95% CI ‐0.15 to 0.08); P = 0.56; 267 participants; 3 trials with 4 treatment groups; low quality evidence). There was moderate heterogeneity (I2 = 37%); results were similar with a fixed‐effect meta‐analysis. There was a high risk of attrition bias in these three studies; we imputed SDs for two of these trials (Boutelle 2011; Collins 2011). In one of the two trials that did not report SDs, there was a reduction in BMI z score in both intervention groups (‐0.3 with parent‐only versus ‐0.14 with parent‐child) (Munsch 2008) . In the study by Golan 2006, there was a reduction in BMI z score in the parent‐only group (‐1.28) and a slight increase in BMI z score in the parent‐child group (0.032).
1.2. Analysis.
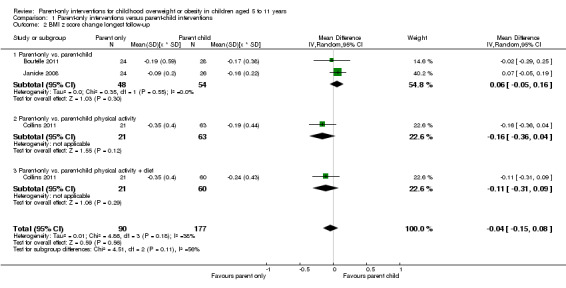
Comparison 1 Parent‐only interventions versus parent‐child interventions, Outcome 2 BMI z score change longest follow‐up.
Two trials also reported the change in percentage overweight at follow‐up. In the study by Golan 2006, immediately after the intervention the overweight change in the parent‐only group was ‐10% (SD 22) and in the parent‐child group this was ‐3% (SD 18) (P < 0.05 for between‐group difference). At follow‐up 12 months later, the change in percentage overweight was ‐12% in the parent‐only group and 0.4% in the parent‐child group (P < 0.05). In Munsch 2008, the change reported was a ‐4% reduction at the end of treatment in the parent‐only group and a ‐2.% reduction at the end of treatment in the parent‐child group (P not significant). At follow‐up six months later, the reduction in per cent overweight was ‐5% in the parent‐only group and ‐2% in the parent‐child group (P not significant).
Collins 2011 reported body weight, adjusted for age, immediately after the intervention and at the longest period of follow‐up (24 months). At six months (immediately after the intervention), in the parent‐only group the change from baseline weight was 0.4 kg (SD 2); in the parent‐child physical activity group this was 1.3 kg (SD 3.2) and in the parent‐child physical activity plus diet group this was 0.1 kg (SD 2.9). At 24 months, in the parent‐only intervention this was ‐1.7 kg (SD 9.4), in the parent‐child physical activity group this was 0.4 kg (SD 11) and in the parent‐child physical activity plus diet group this was ‐0.9 kg (SD 10.7).
Three trials reported parental BMI (Boutelle 2011; Janicke 2008; Munsch 2008). The change from baseline parental BMI in the Janicke 2008 study was ‐0.9 (SD 1.6) in the parent‐based group and ‐0.7 (SD 1.0) in the control group at the end of the intervention (five months) and was ‐0.6 (SD 2.4) in the parent‐only group and 0.2 (SD 1.5) in the control group at the follow‐up at 11 months. There were no substantial differences between groups at either time point (P = 0.93 at four months, P = 0.17 at 10 months). In the study by Munsch 2008, the change in parental BMI at the end of treatment (10 weeks) was 0.1 (SD not calculable) in the parent‐only group and ‐0.04 (SD not calculable) in the parent‐child group. At the end of follow‐up (six months later), the change in parental BMI was ‐0.1 (SD not calculable) in the parent‐only group and 0.1 (SD not calculable) in the parent‐child group. The study reported that there were no significant differences between groups. Boutelle 2011 reported BMI post‐intervention (five months) and six months later. Parental BMI change was ‐0.7 (SD 8.7) in the parent‐only group and ‐0.1 (SD 7.4) in the parent‐child group post intervention, and 0.1 (SD 9.1) in the parent‐only group and 0.3 (SD 7.2) in the parent‐child group at follow‐up. Results were suggested by the authors to be likely to be non‐inferior. There were high levels of non‐completion in these trials.
Adverse events
No trials reported adverse events.
Secondary outcomes
Health‐related quality of life and self esteem
No trials reported health‐related quality of life measures.
Immediately post the four‐month intervention Janicke 2008 assessed the self esteem of the children on four subscales of the validated Self‐Perception Profile for Children (SPPC) measure. These were social self esteem, athletic self esteem, physical self esteem and global self esteem. Results were reported for boys and girls separately because of gender differences in self esteem and, therefore, normative data were presented for the separate groups. An increase in score on this measure corresponds to increased self esteem. Changes from baseline were generally positive for all measures for both the parent‐only and parent‐child groups. There were no substantial differences between the groups seen at follow‐up (analysis was for a treatment effect between the three interventions included in the study, no pairwise analysis was undertaken of the parent‐only and parent‐child groups).
All‐cause mortality
No trials reported all‐cause mortality.
Morbidity
No trials reported morbidity.
Measures of body fat distribution
One trial reported waist circumference adjusted for age and gender (waist z score) (Collins 2011). The change from baseline between groups at the immediate follow‐up was: parent‐only ‐0.3 (SD 0.3); parent‐child physical activity ‐0.1 (SD 0.5); parent‐child physical activity plus diet ‐0.2 (SD 0.5). At 24‐month follow‐up, change from baseline was: parent‐only ‐3.9 (SD 9.9); parent‐child physical activity ‐1.5 (SD 11.6); parent‐child physical activity plus diet ‐1.1 (SD 11.1). Both sets of results should be interpreted in view of the differential and high rates of study non‐completion.
Behaviour change
One trial used the Physical Activity Questionnaire for Older Children (Boutelle 2011). This was a seven‐day recall measure designed to assess physical activity levels and consists of nine items, each being rated on a 5‐point scale. At post‐intervention follow‐up and six months later, results for the parent‐only group showed non‐inferiority to the parent‐child group. Scores were 2.8 (SD 0.6) in the parent‐only group and 2.7 (SD 1) in the parent‐child group at immediate follow‐up, and 4.2 (SD 3.7) in the parent‐only group versus 2.8 (SD 0.7) in the parent‐child group at follow‐up six months later. Non‐completion rates were high in both groups in this study.
Munsch 2008 reported outcomes on the German version of the Child Behaviour CheckList (CBCL), reporting the global score and the subscales of CBCL externalising, CBCL internalising and CBCL social problems at the end of treatment (10 weeks) and six months later. There were no substantial differences between the parent‐only group and parent‐child groups on any of these scales (data provided by study author).
The study by Collins 2011 used an objective measure of physical activity from the Actigraph 7164 uniaxial accelerometer to assess counts per minute over an eight‐day period. The total physical activity recorded increased in all groups at the immediate point of assessment (six months) but there were no substantial differences between groups. At the longest point of follow‐up (12 months), the physical activity recorded increased in the two parent‐child groups and decreased in the parent‐only group, but there were no substantial differences seen. In addition, this study measured parental report of screen behaviours by a validated measure, the Children's Leisure Activities Study Survey. The total screen time use decreased in all three groups at both measurements, but there were no substantial differences between groups.
Participants' views of the intervention
The study by Janicke 2008 asked parents whether they would be prepared to join the programme again. In the parent‐only group, 88% of parents responded that they would and 12% responded that they may be prepared to join the programme again. In the parent‐child intervention, 91% of parents responded that they would and 9% responded that they may be prepared to join the programme again. Children in the parent‐child group were asked if it was true that "Overall, this was a good program", where 85% responded 'really true'; 12% responded 'sort of true' and 3% responded 'sort of not true'.
Parent‐child relationship or assessment of parenting
No trials reported outcomes assessing parent‐child relationships or an assessment of parenting.
Socioeconomic effects
No trials reported outcomes assessing socioeconomic effects.
Parent‐only interventions versus waiting list controls
Six trials reported eight comparisons of a parent‐only intervention and a waiting list control (Aragona 1975; Golley 2007; Janicke 2008; Jansen 2011; Moens 2012; West 2010). To allow consideration of the effects of the interventions, we considered outcomes from the longest period of follow‐up and any post intervention follow‐up. The period for the post intervention follow‐up in these trials ranged from three to six months and the period for the longest point of follow‐up ranged from six to 12 months. Two trials did not report a period of follow‐up beyond the post intervention follow‐up (Moens 2012; West 2010). Losses to follow‐up ranged from 6% to 40% at the post intervention follow‐up and 18% to 60% at the longest period of follow‐up (see Table 5). In the trials by Janicke 2008, Jansen 2011, West 2010, and Aragona 1975, there was a differential rate of losses to follow‐up between groups. In the trials by Golley 2007 and Aragona 1975, losses to follow‐up at both time points were high: between 14% and 24% in the study by Golley 2007 and up to 69% in the study by Aragona 1975. Aragona 1975 had a very small sample size of five participants per treatment group. These factors need to be considered when interpreting the results of the trials.
Primary outcomes
Changes in body mass index and body weight
Three trials reported BMI variables at follow‐up post intervention (Janicke 2008 at four months; West 2010 at 12 weeks; Jansen 2011 at three months). In two trials, this was the BMI z score (Janicke 2008; West 2010), and in the third trial, this was the BMI percentile (Jansen 2011). A fourth study reported individual participant data for weight and height and we calculated the mean BMI from these data (Aragona 1975). Another study reported adjusted BMI based on parental report of weight and height and as the data were, therefore, not reliable, we have not discussed them further here (Moens 2012).
The meta‐analysis for the change in the BMI z score comparing the parent‐only group and the waiting list control group showed an MD of ‐0.12 ((95% CI ‐0.21 to ‐0.04); P = 0.003; 153 participants; 2 trials; low quality evidence; Analysis 2.1). Janicke 2008 had a high risk of attrition and reporting bias, and West 2010 had a high risk of selection and performance bias. We imputed SDs for West 2010.
2.1. Analysis.
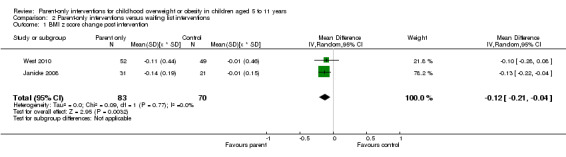
Comparison 2 Parent‐only interventions versus waiting list interventions, Outcome 1 BMI z score change post intervention.
At the longest point of follow‐up, four trials (six comparisons) reported BMI variables (Golley 2007 12 months post baseline; Janicke 2008 and Jansen 2011 six months after; Aragona 1975 39 weeks after). In two trials, this was the BMI z score (Golley 2007; Janicke 2008), in one trial, this was the BMI percentile (Jansen 2011), and in one trial this was the BMI (Aragona 1975).
Meta‐analysis for the change in BMI z score comparing the parent‐only group with the waiting list control group showed a MD of ‐0.10 ((95% CI ‐0.19 to ‐0.01); P = 0.04; 136 participants; 2 trials with 3 treatment arms; low quality evidence; Analysis 2.2). Janicke 2008 had a high risk of attrition and reporting bias, Golley 2007 had a high risk of reporting bias.
2.2. Analysis.
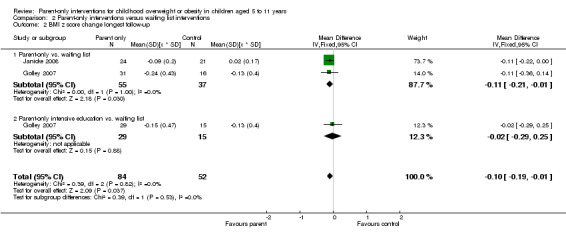
Comparison 2 Parent‐only interventions versus waiting list interventions, Outcome 2 BMI z score change longest follow‐up.
Jansen 2011 found no significant difference between the parent‐only intervention and the waiting list control in BMI percentile change from baseline (MD ‐1.90 (95% CI ‐3.76 to ‐0.04); Analysis 2.4). Aragona 1975 found no significant difference between either the parent‐only plus reinforcement group or the parent‐only group and the waiting list control on change from baseline BMI (Analysis 2.6). These data were based on small numbers and the rate of non‐completion was high and these data have not been combined in a meta‐analysis.
2.4. Analysis.
Comparison 2 Parent‐only interventions versus waiting list interventions, Outcome 4 BMI percentile change longest follow‐up.
2.6. Analysis.
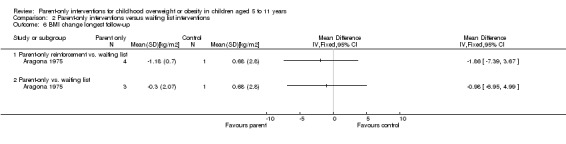
Comparison 2 Parent‐only interventions versus waiting list interventions, Outcome 6 BMI change longest follow‐up.
In Jansen 2011, there was a treatment effect in favour of the parent‐only intervention for BMI percentile change (‐0.48 (95% CI ‐0.89, 0.07); Analysis 2.3); however, Jansen 2011 was at a high risk of bias owing to nine families originally allocated to the waiting list control group being included in the data set for the parent‐only intervention, and, therefore, these data should be considered with caution. In Aragona 1975, there was a reduction from baseline BMI in both the parent‐only plus reinforcement group and the parent‐only intervention group compared with the waiting list control group (Analysis 2.5). However, we did not perform a meta‐analysis because these data were based on small numbers and the rate of non‐completers was high.
2.3. Analysis.
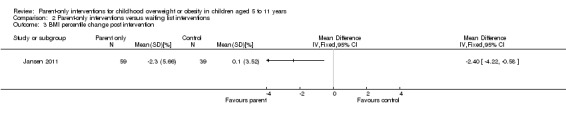
Comparison 2 Parent‐only interventions versus waiting list interventions, Outcome 3 BMI percentile change post intervention.
2.5. Analysis.
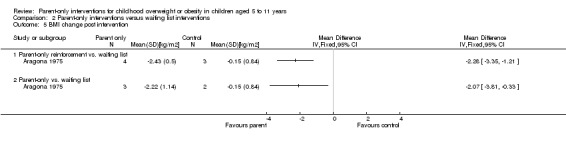
Comparison 2 Parent‐only interventions versus waiting list interventions, Outcome 5 BMI change post intervention.
We undertook no sensitivity analyses on BMI change for trials with high loss to follow‐up as all of the trials had high loss to follow‐up. Therefore, caution is recommended in the interpretation of these data.
Aragona 1975 found a reduction in weight (which we converted to kilograms) in all three intervention groups at the end of the intervention period (parent‐only with reinforcement ‐5.1 kg (SD 0.8); parent‐only without reinforcement ‐4.3 kg (SD 2.9); control ‐0.2 kg (SD 2.1)). At the longest point of follow‐up, there was a decrease in weight in the parent‐only with reinforcement group (‐0.3 (SD 2.4)) and increases in weight in the parent‐only without reinforcement group (3.3 (SD 2.9) and the control group (4.7 (SD 3.4)).
Two trials reported the change in parental BMI (Janicke 2008; Jansen 2011). In the study by Janicke 2008, there was a decrease in parental BMI in both the parent‐only group and the waiting list control group at the post intervention follow‐up (parent‐only ‐0.9 (SD 1.6) versus waiting list control ‐0.7 (SD 2.6); P = 0.93 based on a three‐way comparison). At the longest period of follow‐up (at 10 months), there was also a decrease in parental BMI from baseline, albeit smaller (parent‐only ‐0.6 (SD 2.4) versus waiting list control ‐0.6 (SD 3.6); P = 0.17, based on a three‐way comparison). In the study by Jansen 2011, the parental BMI decreased in the parent‐only group and increased in the waiting list control group immediately post intervention (parent‐only ‐0.3 (SD 4.5) versus waiting list control 0.1 (SD 6.3)), and at the longest period of follow‐up six months post intervention (parent‐only ‐0.2 (SD 4.5) versus waiting list control 0.1 (SD 6.3)).
Adverse events
No trials reported adverse events.
Secondary outcomes
Health‐related quality of life and self esteem
No trials reported health‐related quality of life.
The study by Jansen 2011 assessed self esteem on the SPPC. This validated measure comprises of six subscales, two of which were of interest and reported by the study authors (physical appearance, global self worth). Scores for each item range from 1 to 4 with higher scores relating to poorer outcome. At the post treatment assessment (three months), the physical appearance rating from the parent‐only intervention group's children was 15.9 (SD 5) and in the waiting list control this was 16.1 (SD 4.7). Six months later, the ratings were 16 (SD 5.3) for the parent‐only group and 15.7 (SD 4.7) for the waiting list control group. There was no substantial main effect between groups (comparing both groups over both time periods). On the global self worth scale, the parent‐only intervention rating was 18.8 (SD 4.2) and the waiting list control rating was 20.3 (SD 4.2) at the three month post intervention follow‐up. At the final follow‐up, these ratings were 19 (SD 4.9) for the parent‐only group and 20.2 (SD 4.1) for the waiting list control group. There was no substantial main effect between groups (comparing both groups over both time periods).
Janicke 2008 assessed the self esteem of the children on four subscales (social self esteem, athletic self esteem, physical self esteem and global self esteem) of the SPPC measure at the end of the four‐month intervention. Results were reported for boys and girls separately. Changes from baseline were generally positive for all measures for both the parent‐only and waiting list control groups. There were no substantial differences between the groups at follow‐up.
All‐cause mortality
No trials reported all‐cause mortality.
Morbidity
No trials reported morbidity.
Measures of body fat distribution
Golley 2007 calculated the waist circumference z score. At the final follow‐up (at 12 months), the mean change in score was ‐0.3 (SD 0.5) in the parent‐only intense group, ‐0.2 (0.5) in the parent‐only group and ‐0.02 (0.6) in the waiting list control group. These reductions in waist circumference z scores were not substantially different between groups.
Behaviour change
West 2010 reported the Lifestyle Behaviour Checklist (LBC), which is a measure of child weight‐related problem behaviour and includes items on eating behaviours and physical activity, yielding scores on two scales, the LBC problem scale (lower scores indicate better outcome) and the LBC confidence scale (higher scores indicate better outcome). At the post intervention follow‐up at 12 weeks, the LBC problem scores were 59.4 (SD 20.7) in the parent‐only group and 73.8 (SD 19.3) in the waiting list control group. The LBC confidence scores were 204.4 (SD 37.5) in the parent‐only group compared with 165.8 (SD 46.4) in the waiting list control group (P < 0.0125 on both scales in favour of the parent‐only intervention).
Participants' views of the intervention
For the participants' views of the parent‐only interventions in the study by Golley 2007 see 'Parent‐only interventions versus parent‐only interventions' below.
Parent‐child relationship or assessment of parenting
The study by West 2010 reported data from the Parenting Scale, which is a validated measure of parental discipline practices. It has 30 items and parents indicate their tendencies to use specific discipline strategies using 7‐point Likert scales, where 7 indicates a high probability of making the discipline mistake and 1 indicates a high probability of using an effective, alternative discipline strategy. At the post intervention follow‐up at 12 weeks the Parenting Scale scores were 2.7 (SD 0.7) in the parent‐only group and 3.4 (SD 0.5) in the waiting list control group (P < 0.0125 in favour of the parent‐only intervention; low quality evidence).
Socioeconomic effects
No trials reported socioeconomic effects.
Parent‐only interventions versus minimal contact interventions
Seven trials reported 10 comparisons of a parent‐only intervention and a minimal contact control (Esfarjani 2013; Estabrooks 2009; Mazzeo 2014; Resnick 2009; Small 2013; Resnicow 2015; van Grieken 2013). To allow consideration of the effects of the interventions, we considered outcomes from the longest period of follow‐up and any post intervention follow‐up. The period for the post intervention follow‐up in these trials ranged from three to six months in all but two trials (12 months for van Grieken 2013; 24 months for Resnicow 2015), and the period for the longest point of follow‐up ranged from six to 12 months (one study, Esfarjani 2013, did not state the duration of follow‐up). One study had a planned six‐month follow‐up but did not report results as the loss to follow‐up was high (Mazzeo 2014, 68% to 73% loss to follow‐up). Two trials did not report a period of follow‐up beyond the end of the intervention (Resnick 2009; Resnicow 2015). Losses to follow‐up in the included trials ranged from 0% to 34% at the post intervention follow‐up and were between 17% and 38% at the longest period of follow‐up (see Table 5). These need to be considered when interpreting the results of the trials below.
Primary outcomes
Changes in body mass index and body weight
Six trials (eight comparisons) reported BMI variables at follow‐up post intervention. In one study (two comparisons), this was the BMI z score (Estabrooks 2009), and in the remaining trials, this was the BMI percentile (Mazzeo 2014; Resnick 2009; Resnicow 2015; Small 2013) or BMI (Esfarjani 2013). The period for the outcome assessment differed between trials (Esfarjani 2013 at six months; Estabrooks 2009 at 12 to 24 weeks; Mazzeo 2014 at 12 weeks; Resnick 2009 at 18 to 30 weeks; Resnicow 2015 at 24 months; Small 2013 at 17 weeks).
Meta‐analysis for change in BMI z score between the parent‐only groups and the control group showed an MD of ‐0.00 ((95% CI ‐0.08 to 0.08); P = 0.99; 170 participants; 1 trial with 3 treatment arms; low quality evidence). There was a high risk of attrition bias and reporting bias for Estabrooks 2009 and we imputed SDs.
Four trials reported the change in BMI percentile; however, because of lack of standardisation these could not be pooled. None of the studies demonstrated treatment effects (Analysis 3.3).
3.3. Analysis.
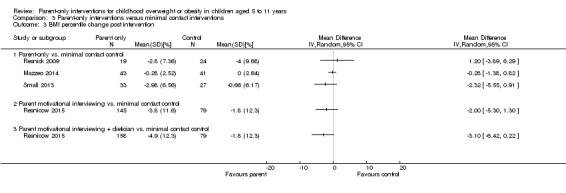
Comparison 3 Parent‐only interventions versus minimal contact interventions, Outcome 3 BMI percentile change post intervention.
For BMI change from the trial by Esfarjani 2013, there was no difference between comparison groups (Analysis 3.5).
3.5. Analysis.
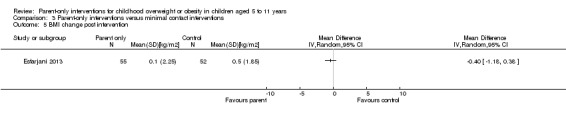
Comparison 3 Parent‐only interventions versus minimal contact interventions, Outcome 5 BMI change post intervention.
At the longest point of follow‐up, four trials (five comparisons) reported BMI variables. In one trial (two comparisons, Estabrooks 2009), this was the BMI z score, in one trial this was the BMI percentile (Small 2013), and in the remaining two trials this was the BMI (Esfarjani 2013; van Grieken 2013). The duration of follow‐up varied, in the study by Estabrooks 2009 and Small 2013, this was six months after the end of the intervention; the study by Esfarjani 2013 did not report the duration of follow‐up after the six month intervention; in the study by van Grieken 2013, follow‐up was at 24 months following an intervention of up to 12 months.
Meta‐analysis for the change in BMI z score comparing the parent‐only group with the minimal contact control group showed a MD of 0.01 ((95% CI ‐0.07 to 0.09); P = 0.81; 165 participants; 1 trial with 3 treatment arms; low quality evidence; Analysis 3.2).
3.2. Analysis.
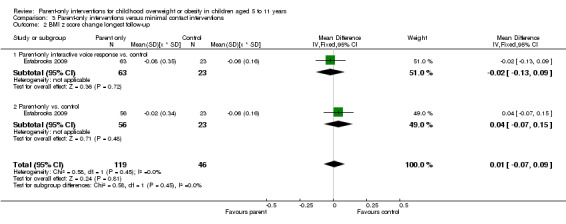
Comparison 3 Parent‐only interventions versus minimal contact interventions, Outcome 2 BMI z score change longest follow‐up.
The one study reporting BMI percentile found no a substantial treatment effect on BMI percentile change (MD ‐0.93 (95% CI ‐3.49 to 1.63); Analysis 3.4).
3.4. Analysis.

Comparison 3 Parent‐only interventions versus minimal contact interventions, Outcome 4 BMI percentile change longest follow‐up.
Meta‐analysis for change from baseline BMI showed an MD of ‐0.12 ((95% CI ‐0.39 to 0.15); P = 0.39; 614 participants; 2 trials; low quality evidence; Analysis 3.6) (Esfarjani 2013; van Grieken 2013). In the cluster trial, the study authors calculated an intra‐cluster correlation coefficient of 0.06 for BMI and the numbers analysed appear to have been adjusted appropriately (van Grieken 2013). All of these trials had high loss to follow‐up, which should be considered when interpreting these results.
3.6. Analysis.
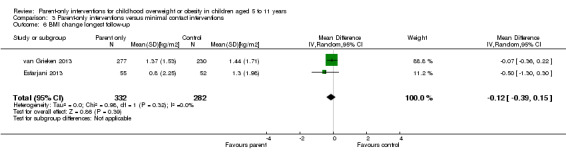
Comparison 3 Parent‐only interventions versus minimal contact interventions, Outcome 6 BMI change longest follow‐up.
No trials reported the parental BMI.
In the study by Esfarjani 2013, weight in kilograms was also reported as an outcome. The change from baseline weight at the end of the six‐month intervention was 1.8 kg (SD 4.9) in the parent‐only group and 2.6 kg (SD 5) in the control group, giving a difference of 0.8 kg (95% CI ‐2.67 to 1.1). At the longest point of follow‐up (not defined), the weight change for the two groups were 5 kg (SD 5.1) for the parent‐only group and 5.7 kg (SD 5.17) for the control group (difference 0.7 kg (95% CI ‐2.6 to 1.2).
Adverse events
No trials reported adverse events.
Secondary outcomes
Health‐related quality of life and self esteem
One study reported narratively that there were no improvements in health‐related quality of life but reported no data (Mazzeo 2014).
No trials reported self esteem.
All‐cause mortality
No trials reported all‐cause mortality.
Morbidity
No trials reported morbidity.
Measures of body fat distribution
Small 2013 reported waist and waist‐to‐height ratio. At the post intervention assessment (three months), waist circumference was 28.2 inches (SD 3.8) in the parent‐only intervention compared with 28.4 inches (SD 3.7) in the control group (1 inch = 2.5 cm). At the final assessment (six months later), the waist circumference in the parent‐only group was 29.5 inches (SD 3.5) compared with 28.9 inches (3.7) in the control group. The waist‐to‐height ratio at three months was 0.58 (SD 0.08) in the parent‐only group and 0.60 (SD 0.06) in the control group. The corresponding values at six‐month follow‐up were 0.59 (SD 0.09) in the parent‐only group and 0.59 (SD 0.08) in the control group. The trial reported that these results were not statistically significant between groups. The study by Esfarjani 2013 reported waist circumference. The change from baseline waist circumference at the end of the six‐month intervention was ‐1.0 cm (SD 5.8) in the parent‐only group and 1.5 cm (SD 5.1) in the control group, with a difference of 2.5 cm (95% CI ‐4.6 to ‐0.4). At the longest point of follow‐up (not defined), the changes in waist circumference were 2 cm (SD 5.8) in the parent‐only group and 3.5 cm (SD 5.28) in the control group, with a difference of 1.5 cm (95% CI ‐3.6 to 0.6). van Grieken 2013 also reported waist circumference at 24‐month follow‐up. In the parent‐only group the change in waist circumference was 7.2 cm (SD 5.5) compared with 7.3 cm (SD 5.3) in the control group, with a difference of 0.1 cm (95% CI ‐1.1 to 0.8).
Esfarjani 2013 reported hip circumference. There was a change in hip circumference in the parent‐only group at the end of the intervention of ‐0.5 cm (SD 4.9) compared with 1.4 cm (SD 4.9) in the control group, with a difference of 1.9 cm (95% CI ‐3.8 to ‐0.1). At the longest point of follow‐up (not defined), the change in hip circumference was 2.1 cm (SD 4.8) in the parent‐only group and 3.7 cm (SD 5.1) in the control group, with a difference of 1.6 cm (95% CI ‐3.5 to 0.2).
Behaviour change
No trials reported validated measures assessing behaviour change.
Participants' views of the intervention
The study by Mazzeo 2014 sought parental views at completion of the parent‐only intervention. Parents either strongly agreed (79%) or moderately agreed (21%) with the statement "I enjoyed attending each NOURISH session." In addition, 92% strongly agreed that they would recommend the intervention to other parents, 91% strongly or moderately agreed that the sessions had helped them eat in a healthier manner and 78% said they were exercising more. The study also reported examples of qualitative responses received from parents in interviews, both positive and negative (e.g. "I really enjoyed them and hearing what other parents concerns were like mine" and "I was excited at first but once it started, it was sometimes difficult to get there, park, and stay focused for 90 min after working all day").
Resnick 2009 asked parents a questions about the materials used and both groups appeared to be satisfied with the materials. Of 20 parents in the parent‐only group, 13 (65%) reported reading all the study materials, the corresponding rate in the control group was 17/22 (77%). Some 17 (94%) parents in the parent‐only group and 18 (82%) of parents in the control group stated that they would recommend the programme to other families. The parents in the intervention group were also asked if they found the community health workers to be helpful and 16/20 (80%) stated "yes". The trial authors reported responses to other questions.
Parent‐child relationship or assessment of parenting
Mazzeo 2014 reported parental concern about the child's weight. This was one of seven subscales of the Child Feeding Questionnaire (results of other subscales were not reported). Parental concern decreased from 4.7 (SD 0.5) to 4.6 (SD 0.7) in the parent‐only group and from 4.7 (SD 0.5) to 4.7 (SD 0.5) in the control group at the follow‐up immediately following the 12‐week intervention (P = 0.041 in favour of the parent‐only intervention; 93 participants; 1 trial; low quality evidence).
Socioeconomic effects
No trials reported socioeconomic effects.
Parent‐only interventions versus parent‐only interventions
Seven trials reported nine comparisons of two different parent‐only interventions (Aragona 1975; Estabrooks 2009; Golley 2007; Magarey 2011; Raynor 2012a; Raynor 2012b; Resnicow 2015). The point of post intervention follow‐up was three months in one study (Aragona 1975); six months in five studies (Estabrooks 2009; Golley 2007; Magarey 2011; Raynor 2012a; Raynor 2012b); and 24 months in one study (Resnicow 2015). The longest points of follow‐up ranged from 12 to 24 months (all trials except Magarey 2011 had a 12‐month follow‐up, Resnicow 2015 only had one period of follow‐up at the end of the intervention). Losses to follow‐up in the included trials ranged from 3% to 40% at the post intervention follow‐up and were between 8% and 60% at the longest period of follow‐up (see Table 5). These need to be considered when interpreting the results of the trials.
Primary outcomes
Changes in body mass index and body weight
Five trials reported BMI z score and Analysis 4.1 and Analysis 4.2 show the change from baseline for each study. No meta‐analysis was possible because there was little or no consistency between trial interventions and comparators. A narrative synthesis of the results of the trials follows.
4.1. Analysis.
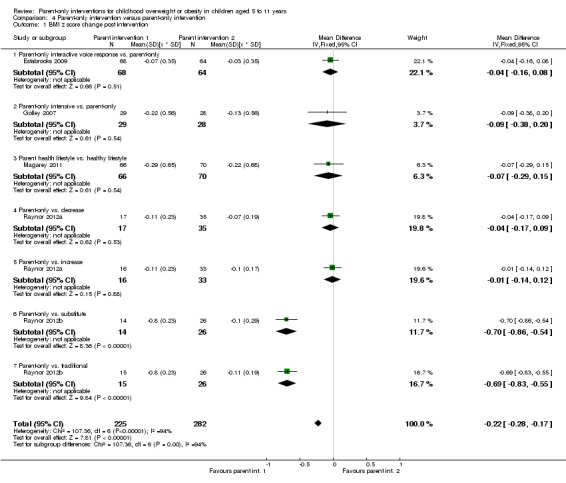
Comparison 4 Parent‐only intervention versus parent‐only intervention, Outcome 1 BMI z score change post intervention.
4.2. Analysis.
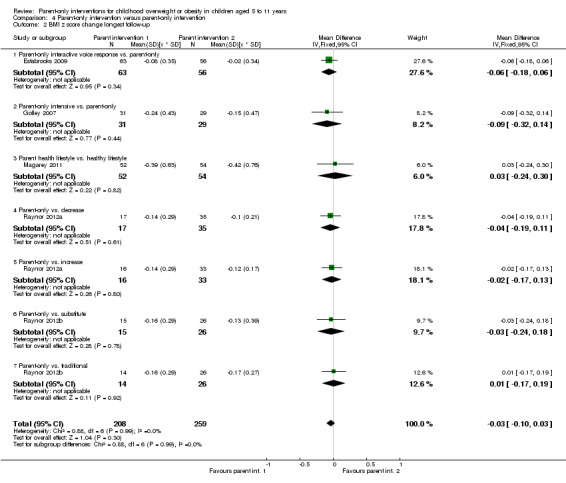
Comparison 4 Parent‐only intervention versus parent‐only intervention, Outcome 2 BMI z score change longest follow‐up.
The study by Estabrooks 2009 (a three‐arm trial, see above for other comparisons) compared two parent‐only interventions. Parents either attended a group intervention that addressed behavioural health skills and knowledge or a group intervention that addressed the same issues but also included 10 automated telephone counselling sessions over a period of six months. Both groups also utilised a workbook aimed at promoting healthy habits and physical activity throughout the interventions. Change in BMI z score at the immediate follow‐up at six months showed an MD of ‐0.04 ((95% CI ‐0.16 to 0.08); P = 0.51) and at the follow‐up 12 months later an MD of ‐0.06 ((95% CI ‐0.18 to 0.06); P = 0.34). Losses to follow‐up were above 20% in both groups at both time points.
In a three‐arm study, Golley 2007 had two parent‐only interventions: parents either attended a behavioural change parenting skills training intervention or the same intervention with seven additional intensive lifestyle support group sessions. At six months, BMI z score change showed a MD of ‐0.09 ((95% CI ‐0.38 to 0.20); P = 0.54) where there was a 24% drop‐out rate in both groups at this time point. Similarly, at 12 months' follow‐up, the MD was ‐0.09 ((95% CI ‐0.32 to 0.14); P = 0.44). The non‐completion rate at this point of follow‐up was 18% in the intensive group and 22% in the standard group intervention.
Magarey 2011 compared two parent‐only interventions, a healthy lifestyle group (which included recommendations, practical skills and monitoring aspects) and a group that had the same healthy lifestyle intervention with the addition of four parenting skills sessions. BMI z score change at the completion of the intervention at six months showed an MD of ‐0.07 ((95% CI ‐0.29 to 0.15); P = 0.54) and at the 24‐month follow‐up, an MD of 0.03 ((95% CI ‐0.24 to 0.30); P = 0.82). Non‐completion rates in this study were between 17% and 22% at the post intervention follow‐up and 36% and 39% at the 24‐month follow‐up.
One publication reported two trials and each trial had three arms, a standard arm that was a growth monitoring intervention and two additional parent‐only interventions. In the first trial, the two additional interventions focused on diet, with one intervention focusing on decreasing sugar and salty snack food and drink consumption and the second intervention focusing on increasing fruit, vegetable and low‐fat dairy intake (Raynor 2012a). In the second trial, the two additional interventions focused on diet and physical activity, with one intervention focusing on decreasing sugar intake and increasing physical activity and the second intervention focusing on increasing low‐fat intake and decreasing sedentary lifestyles such as watching television (Raynor 2012b). The completion rates in these trials were better than the other trials, with between 3% and 12% of participants not completing the study. At the point of completion of the intervention (six months) in the Raynor 2012a trial, the difference in BMI z score change between the growth monitoring group and the group focusing on decreasing sugar consumption was ‐0.04 ((95% CI ‐0.17 to 0.09); P = 0.53; data provided by study authors). The difference between the growth monitoring group and the group focusing on increasing healthy food consumption was ‐0.01 ((95% CI ‐0.14 to 0.12); P = 0.88; data provided by study authors). Similar results were also seen at 12 months in the decreasing sugar group with an MD of ‐0.04 ((95% CI ‐0.19 to 0.11); P = 0.61), and in the increasing healthy food group with an MD of ‐0.02 ((95% CI ‐0.17 to 0.13); P = 0.80) (data provided by study authors). At the point of completion of the Raynor 2012b trial, BMI z score change between the group focusing on increasing activity compared with the growth monitoring group was ‐0.69 ((95% CI ‐0.83 to ‐0.55); P < 0.00001; data provided by study authors). The difference between the group focusing on reducing sedentary behaviours compared with the growth monitoring group was ‐0.70 (( 95% CI ‐0.86 to ‐0.54); P < 0.00001; data provided by study authors). At 12 months, there was a different pattern, with neither comparisons showing substantial differences between groups: increasing activity group MD 0.01 ((95% CI ‐0.17 to 0.19); P = 0.92) and decreasing sedentary behaviours group MD ‐0.03 ((95% CI ‐0.24 to 0.18); P = 0.78) (data provided by study authors).
Aragona 1975 reported the weight and height of individual participants and mean BMI was calculated from these data. Both parent‐only interventions included training in nutrition, exercise and behaviour, and parents contracted to the investigators regarding a weight loss goal for their child. One intervention also taught reinforcement techniques. Immediately after the intervention, and at the longest point of follow‐up, there were no substantial differences in change from baseline BMI seen (Analysis 4.3; Analysis 4.4).
4.3. Analysis.
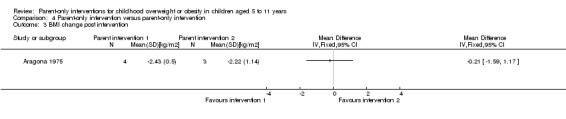
Comparison 4 Parent‐only intervention versus parent‐only intervention, Outcome 3 BMI change post intervention.
4.4. Analysis.
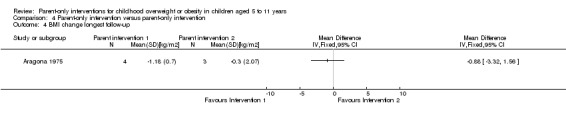
Comparison 4 Parent‐only intervention versus parent‐only intervention, Outcome 4 BMI change longest follow‐up.
The study by Resnicow 2015 reported the BMI percentile at the end of the 24‐month intervention. There were no differences between the two parent‐only interventions (Analysis 4.5).
4.5. Analysis.
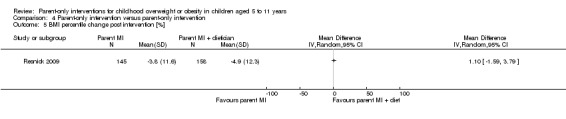
Comparison 4 Parent‐only intervention versus parent‐only intervention, Outcome 5 BMI percentile change post intervention [%].
Adverse events
The trials by Raynor 2012a; Raynor 2012b reported narratively that there were no serious adverse events.
Secondary outcomes
Health‐related quality of life and self esteem
No trials reported health‐related quality of life or self esteem.
All‐cause mortality
No trials reported all‐cause mortality.
Morbidity
No trials reported morbidity.
Measures of body fat distribution
Golley 2007 calculated waist circumference z score. At the post intervention follow‐up (six months), the mean change in waist z score was ‐0.27 (SD 0.7) in the parent‐only intense group and ‐0.12 (SD 0.61) in the parent‐only group. These reductions in waist circumference z scores were not significantly different between groups. At the final follow‐up (at 12 months), the mean change in score was ‐0.31 (SD 0.53) in the parent‐only intense group and ‐0.17 (0.50) in the parent‐only group. These reductions in waist circumference z scores were not statistically significantly different between groups.
Behaviour change
No trials reported behaviour changes.
Participants' views of the intervention
Golley 2007 reported a programme evaluation. Response rates varied, with 26/31 in the parenting and intensive lifestyle group responding and 10/29 in the parenting group responding. All participants in both groups rated the quality of the service provided during the interventions as good to excellent. Fifty per cent (13/26) of participants in the parenting and intensive lifestyle training group and 80% (8/10) in the parent‐only group said they either generally or definitely received the type of help they required from the respective programme. One hundred per cent of parents in the parenting group and 85% (22/26) in the parenting and intensive lifestyle group stated they were satisfied to very satisfied with the amount of help received. One hundred per cent of parents in the parenting group and 92% (24/26) in the parenting and intensive lifestyle group stated they were helped somewhat or helped a great deal by the intervention. The study authors provided other responses.
Parent‐child relationship or assessment of parenting
One study reported results from the Alabama Parenting Questionnaire (satisfaction, efficacy, involvement, positive parenting, poor monitoring, inconsistent discipline, corporal punishment), which is a validated measure (Magarey 2011). However, outcomes were reported only for the total group, and no comparisons between the two treatment groups were provided.
Socioeconomic effects
No trials reported socioeconomic effects.
Subgroup analyses
We did not perform subgroups analyses because there were not enough trials to estimate effects in various subgroups.
Sensitivity analyses
We did not perform any sensitivity analyses because there were not enough trials included in the analyses.
Assessment of reporting bias
We did not draw funnel plots due to limited number of trials with data included in any one analysis.
Ongoing trials
We found 10 ongoing RCTs (see Characteristics of ongoing studies table). Nine of these RCTs are parallel trials, one is a cluster trial. The ages of the participants in these trials incorporate the range of 2 to 6 years in six trials, 7 to 13 years in two trials and were not reported in three trials. In five trials, the target population are children classed as overweight (various definitions), in two trials the target population are children classed as obese, and in four trials the population is described as overweight or obese. Five trials are comparing a parent‐only intervention with another parent‐only intervention; three trials are comparing a parent‐only intervention with a parent‐child intervention; three trials are comparing a parent‐only intervention with a minimal contact intervention and one trial is comparing a parent‐only intervention to no treatment. The primary outcome is the BMI z score in nine of the 10 ongoing trials; in the one other trial, this is weight change. The estimated study completion dates, where reported, range from January 2014 to November 2016.
Discussion
Summary of main results
This systematic review summarised 20 RCTs examining the effect of parent‐only interventions for treating overweight and obesity in children aged 5 to 11 years. We only included trials with at least a six‐month outcome assessment with the aim of assessing the longer‐terms effects of these types of interventions. Interventions and comparators varied between the included trials and we divided the trials into four main groups to ease interpretation. Outcomes assessed also varied between groups; although all trials reported a measure of body weight, this varied between the BMI, BMI z score, BMI percentile and weight. Many trials were of low quality, non‐completion rates of the trials were generally high and few trials accounted for these in their analyses. To allow comparisons across trials, we analysed outcome data, where reported, from the post intervention assessment and the longest period of follow‐up.
Overall, in trials comparing a parent‐only intervention with a parent‐child intervention there were no substantial differences in BMI measures at either the post intervention follow‐up or the longest follow‐up period. Other outcomes, reported less consistently across trials, also generally reported no substantial differences between groups, including parental BMI, behavioural changes and health‐related quality of life. One study undertook a process evaluation where parents responses about undertaking the programme again were similar across groups. In trials comparing a parent‐only intervention with a waiting list control, there was a treatment effect on BMI in favour of the parent‐only intervention at the post intervention follow‐up and at the longest follow‐up period (low quality evidence). There was no substantial effect of the parent‐only intervention on parental BMI or the child's self esteem. One trial reported better outcomes in the parent‐only groups on a measure of behavioural change and on a measure of parenting discipline practices (low quality evidence). There were no substantial effects of parent‐only interventions on BMI or weight when compared with minimal contact control interventions. One trial found reduced levels of parental concern in the parent‐only group. Two trials reported process evaluations and the interventions were generally rated more highly by those who responded in the parent‐only group than the control group. A number of trials compared a parent‐only intervention with another parent‐only intervention. To meet the inclusion criteria for the review, parent‐only comparisons were required to be a concomitant therapy delivered in the intervention arm. There were few similarities between interventions and comparators across the included trials. Generally, these trials did not show substantial differences between their respective parent‐only groups on the various outcomes reported.
All results need to be interpreted cautiously because of the low quality of these trials and the differences between the interventions and comparators.
Overall completeness and applicability of evidence
This review included only trials that reported outcomes at six months or more; however, within the individual trials there was considerable heterogeneity with the duration of the interventions and their respective follow‐ups varied considerably. Few trials had similar intervention characteristics and, together with the mixture of outcomes assessed and results seen, it was difficult to establish whether there is any particular intervention type that is more likely to lead to a successful outcome. It was our intention to explore the impact of the type of parent‐only intervention (e.g. focusing on parenting, cognitive behavioural therapy, behaviour change) and the setting (e.g. community, clinic‐based, internet), to determine if any specific approach was more effective for the treatment of childhood overweight and obesity. Despite the relatively large number of trials included in the review, the profound clinical heterogeneity meant that we were unable to do this. Few trials provided enough details of the intervention to allow these to be replicated by other trialists (length, type, nature of sessions, group sizes, contents, training of the provider or theoretical basis for the intervention). Few trials reported outcomes over a relatively long period of follow‐up (e.g. over two years).
Results seemed to suggest that parent‐only interventions are similar to parent‐child interventions, and minimal contact interventions, but that they are better than a waiting list control. However, there are numerous issues to consider, not least the sample sizes of many trials, the loss to follow‐up and the low quality evidence.
There was an insufficient number of trials reporting secondary outcomes of interest to the review and we could only pool BMI indices in meta‐analyses.
Quality of the evidence
Trial quality was generally low with a large proportion of trials being rated at high risk of bias on individual risk of bias criteria, and many others being rated at unclear risk of bias. GRADE assessment of the outcomes that have been pooled in this review have led to trials being downgraded for risk of bias (in particular attrition bias), and also imprecision owing to the small number of trials and small sample sizes. This makes overall interpretation of the data difficult.
Potential biases in the review process
We conducted a comprehensive search across major databases for interventions involving parent‐only interventions. In addition, we screened the reference lists of systematic reviews. Two review authors comprehensively selected, assessed trials; extracted data and assessed quality of trials for inclusion to minimise potential biases in the review processes. No decisions were made about the analysis or investigation of heterogeneity after seeing the data. Where data of relevance were missing, either to allow assessment of eligibility or at the data extraction stage, the review authors contacted the study authors for further information. Multiple groups were included in seven of the included studies and for analyses we split the shared group into two or more groups with smaller sample sizes. This approach only partially overcomes a unit of analysis error as the resulting comparisons remain correlated. Three trials are awaiting classification as information is currently unavailable to the review.
Agreements and disagreements with other studies or reviews
National Institute for Health and Care Excellence (NICE) guidance emphasises the importance of parental support in weight management services for children and young people under the age of 18 years (NICE 2013). However, parent‐only interventions are not specifically mentioned, instead family‐based interventions are advocated. The results are consistent with a previous meta‐analysis that has compared parent‐only with parent‐child (family‐focused) interventions, showing no substantial difference in the change in the BMI z score between groups (Jull 2013). The findings also agree with another systematic review that indicated that parent‐only interventions were at least as effective as parent‐child interventions, albeit showing higher drop‐out from the parent‐only interventions (Ewald 2014). Jang 2015 have also considered parent‐only interventions in a systematic review using the RE‐AIM ( Reach Effectiveness Adoption Implementation Maintenance) framework to analyse reach, adoption, implementation, efficacy/effectiveness and maintenance. They also support the effectiveness of parent‐only interventions in improving children's BMIs, but that interventions did not appear to reach higher‐risk populations. The current systematic review, as far as we are aware, is the first to analyse the parent‐only interventions by comparison group, comparing with parent‐child interventions, with waiting list and minimal contact interventions, and with different parent‐only interventions.
Authors' conclusions
Implications for practice.
Parent‐only interventions may be an effective treatment option for overweight or obese children aged 5 to 11 years when compared with waiting list controls; however, parent‐only interventions had similar effects compared with parent‐child interventions and compared with those with minimal contact controls. However, the evidence for parent‐only interventions is at present limited, particularly when the trials were split for analysis by comparator, and some of the trials had a high risk of bias.
Implications for research.
The systematic review identified 10 ongoing trials that have a parent‐only intervention arm, which will contribute to the results of this review when being updated. These trials will improve the robustness of the analyses by type of comparator, and may permit subgroup analysis by intervention component and the setting for parent‐only interventions.
There is a need to conduct and report cost‐effectiveness analyses in these ongoing trials in order to establish whether parent‐only interventions are more cost‐effective than parent‐child interventions. Trial reports should provide adequate details about the interventions to be replicated by others and report important outcomes such as health‐related quality of life.
History
Review first published: Issue 12, 2015
| Date | Event | Description |
|---|---|---|
| 11 October 2008 | New citation required and conclusions have changed | This review concludes that combined behavioural lifestyle interventions compared to standard care or self‐help can produce a significant and clinically meaningful reduction in overweight in children and adolescents. The search was updated to May 2008. Some amendments were made to update the search strategies. No changes have been made to other aspects of the methodology. Forty‐six new studies have been included. These included information on drug interventions for treating obesity in adolescents. The added evidence suggests that lifestyle interventions appear to have positive effects in the treatment of child and adolescent obesity. Furthermore, orlistat and sibutramine were found to have beneficial effects on adiposity in obese adolescents. However, a range of adverse effects was noted. |
| 3 July 2008 | Amended | Converted to new review format. Authorship changed with new authors and new contact person. |
Notes
Part of the 'Background', Methods' section, appendices, additional tables and figures 1 to 3 of this review are based on a standard template established by the Cochrane Metabolic and Endocrine Disorders Group.
Acknowledgements
We thank Drs Janicke, Moens, Raynor, Roth and Van Grieken for providing additional information about their trials (Janicke 2008; Moens 2012; Munsch 2008; Raynor 2012a; van Grieken 2013). We thank Christine Clar for translating a German publication to confirm its status as an ongoing study (subsequently excluded following contact with the authors). We acknowledge the editorial contributions by Gudrun Paletta and Bernd Richter (Cochrane Metabolic and Endocrine Disorders Group).
Appendices
Appendix 1. Search strategies
| Cochrane Library |
|
Part I: Obesity 1. [mh ^Obesity] 2. [mh ^"Obesity, Morbid"] 3. [mh ^"Obesity, Abdominal"] 4. [mh ^"Pediatric Obesity"] 5. [mh ^Overweight] 6. [mh ^"Weight Loss"] 7. (adipos* or obes*):ti,ab 8. (overweight* or ("over" next weight*)):ti,ab 9. ("weight" near/1 (reduc* or los* or control* or manage*)):ti,ab 10. {or #1‐#9} Part II: Intervention 11. [mh "Behavior Therapy"] 12. [mh "Counseling"] 13. [mh ^"Family Therapy"] 14. [mh ^"Social Support"] 15. [mh ^"Program Evaluation"] 16. [mh "Exercise"] 17. [mh "Exercise Therapy"] 18. [mh "Physical Education and Training"] 19. [mh "Exercise Movement Techniques"] 20. [mh ^"Motor Activity"] 21. [mh Diet] 22. [mh "Diet Therapy"] 23. [mh ^"Patient Education as Topic"] 24. [mh ^"Health Education"] 25. [mh "Health Behavior"] 26. [mh "Health Promotion"] 27. [mh ^"School Health Services"] 28. [mh ^"School Nursing"] 29. [mh ^"Life style"] 30. (("obesity" near/4 "intervention") or "program" or "programme" or "camp" or "camps"):ti,ab 31. ("lifestyle" or "life style"):ti,ab 32. exercis*:ti,ab 33. (physic* next (activ* or fit*)):ti,ab 34. (walk* or jog* or swim* or ("weight" next lift*) or danc* or "aerobics"):ti,ab 35. ((physic* or strength* or resist* or "circuit" or "weight" or aerob* or "cross" or "endurance" or structur*) near/4 train*):ti,ab 36. ("behavioral" or "behavioural" or (("behavior" or "behaviour") next "modification") or psychoth* or "psychosocial"):ti,ab 37. (("group" or "family" or cognit* or behav*) next therap*):ti,ab 38. (counselling or counselling):ti,ab 39. educat*:ti,ab 40. (("parent" or "parents" or "family") next ("based" or "focused" or "directed" or "centered" or "only" or "led")):ti,ab 41. (diet* or "healthy nutrition" or (nutrition* next ("knowledge" or educat* or therap* or program* or intervention*))):ti,ab 42. {or #11‐#41} Part III: Part I + Part II and additional MeSH/subheading combination 43. #10 and #42 44. [mh ^Obesity] or [mh ^"Obesity, Morbid"] or [mh ^Overweight] 45. [mh /DH,PC,RH,TH,PX][diet therapy or prevention & control or rehabilitation or therapy or psychology] 46. #44 and #45 47. #43 or #46 Part IV: Population [adapted fromLeclercq 2013] 48. [mh ^Adolescent] 49. [mh Child] 50. [mh ^Infant] 51. [mh ^Pediatrics] 52. "minors":ti,ab 53. ("boy" or "boys" or "boyhood"):ti,ab 54. girl*:ti,ab 55. ("kid" or "kids"):ti,ab 56. infant*:ti,ab 57. ("baby" or "babies"):ti,ab 58. ("toddler" or "toddlers"):ti,ab 59. ("child" or "childs" or children* or childhood* or childcare* or schoolchild*):ti,ab 60. adolescen*:ti,ab 61. juvenil*:ti,ab 62. youth*:ti,ab 63. (teen* or preteen*):ti,ab 64. (underage* or ("under" next age*)):ti,ab 65. pubescen*:ti,ab 66. (paediatric* or paediatric*):ti,ab 67. {or #48‐#66} Part V: Part III AND IV and additional MeSH/subheading combination 68. #47 and #67 69. [mh ^"Pediatric Obesity"] 70. [mh /DH,PC,RH,TH,PX] 71. #69 and #70 72. #68 or #71 |
| MEDLINE (Ovid SP) |
|
Part I: Obesity 1. Obesity/ 2. Obesity, Morbid/ 3. Obesity, Abdominal/ 4. Pediatric Obesity/ 5. Overweight/ 6. Weight Loss/ 7. (adipos* or obes*).tw. 8. (overweight* or over weight*).tw. 9. (weight adj1 (reduc* or los* or control* or manage*)).tw. 10. or/1‐9 Part II: Intervention 11. exp Behavior Therapy/ 12. exp Counseling/ 13. Family Therapy/ 14. Social Support/ 15. Program Evaluation/ 16. exp Exercise/ 17. exp Exercise Therapy/ 18. exp "Physical Education and Training"/ 19. exp Exercise Movement Techniques/ 20. Motor Activity/ 21. exp Diet/ 22. exp Diet Therapy/ 23. Patient Education as Topic/ 24. Health Education/ 25. exp Health Behavior/ 26. exp Health Promotion/ 27. School Health Services/ 28. School Nursing/ 29. Life style/ 30. ((obesity adj3 intervention) or program or programme or camp?).tw. 31. (lifestyle or life style).tw. 32. exercis*.tw. 33. (physic* adj (activ* or fit*)).tw. 34. (walk* or jog* or swim* or weight lift* or danc* or aerobics).tw. 35. ((physic* or strength* or resist* or circuit or weight or aerob* or cross or endurance or structur*) adj3 train*).tw. 36. (behavio?ral or behavio?r modification or psychoth* or psychosocial).tw. 37. ((group or family or cognit* or behav*) adj therap*).tw. 38. counsel?ing.tw. 39. educat*.tw. 40. ((parent? or family) adj (based or focused or directed or centered or only or led)).tw. 41. (diet* or healthy nutrition or (nutrition* adj (knowledge or educat* or therap* or program* or intervention*))).tw. 42. or/11‐41 Part III: Part I + Part II and additional MeSH/subheading combination 43. 10 and 42 44. Obesity/ or Obesity, Morbid/ or Overweight/ or Weight Loss/ 45. diet therapy.fs. or prevention & control.fs. or rehabilitation.fs. or therapy.fs. or psychology.fs. 46. 44 and 45 47. 43 or 46 Part IV: Population [adapted fromLeclercq 2013] 48. Adolescent/ 49. exp Child/ 50. Infant/ 51. Pediatrics/ 52. minors.tw. 53. (boy or boys or boyhood).tw. 54. girl*.tw. 55. infant*.tw. 56. (baby or babies).tw. 57. toddler?.tw. 58. (kid or kids).tw. 59. (child or childs or children* or childhood* or childcare* or schoolchild*).tw. 60. adolescen*.tw. 61. juvenil*.tw. 62. youth*.tw. 63. (teen* or preteen*).tw. 64. (underage* or under age*).tw. 65. pubescen*.tw. 66. p?ediatric*.tw. 67. or/48‐66 Part V: Part III AND IV and additional MeSH/subheading combination 68. 47 and 67 69. Pediatric Obesity/ 70. diet therapy.fs. or prevention & control.fs. or rehabilitation.fs. or therapy.fs. or psychology.fs. 71. 69 and 70 72. 68 or 71 Part VI: Study filter [Cochrane Handbook 2008 RCT filter ‐ sensitivity and precision maximizing version] 73. randomised controlled trial.pt. 74. controlled clinical trial.pt. 75. randomi?ed.ab. 76. placebo.ab. 77. clinical trials as topic/ 78. randomly.ab. 79. trial.ti. 80. or/73‐79 81. exp animals/ not humans/ 82. 80 not 81 Part VII: Part V + Part VI 83. 72 and 82 |
| EMBASE (Ovid SP) |
|
Part I: Obesity 1. obesity/ 2. morbid obesity/ 3. abdominal obesity/ 4. childhood obesity/ 5. weight reduction/ 6. weight control/ 7. (adipos* or obes*).tw. 8. (overweight* or over weight*).tw. 9. (weight adj1 (reduc* or los* or control* or manage*)).tw. 10. or/1‐9 Part II: Intervention 11. behavior therapy/ 12. cognitive therapy/ 13. exp counselling/ 14. family therapy/ 15. social support/ 16. exp program evaluation/ 17. exp exercise/ 18. exp physical education/ 19. exp physical activity/ 20. exp motor activity/ 21. training/ 22. exp diet/ 23. exp diet therapy/ 24. nutritional health/ 25. child nutrition/ 26. feeding behavior/ 27. patient education/ 28. health promotion/ 29. health literacy/ 30. nutrition education/ 31. health education/ 32. school health education/ 33. school health service/ 34. lifestyle/ 35. lifestyle modification/ 36. ((obesity adj3 intervention) or program or programme or camp?).tw. 37. (lifestyle or life style).tw. 38. exercis*.tw. 39. (physic* adj (activ* or fit*)).tw. 40. (walk* or jog* or swim* or weight lift* or danc* or aerobics).tw. 41. ((physic* or strength* or resist* or circuit or weight or aerob* or cross or endurance or structur*) adj3 train*).tw. 42. (behavio?ral or behavio?r modification or psychoth* or psychosocial).tw. 43. ((group or family or cognit* or behav*) adj therap*).tw. 44. counsel?ing.tw. 45. educat*.tw. 46. ((parent? or family) adj (based or focused or directed or centered or only or led)).tw. 47. (diet* or healthy nutrition or (nutrition* adj (knowledge or educat* or therap* or program* or intervention*))).tw. 48. or/11‐47 Part III: Part I + Part II and additional MeSH/subheading combination 49. 10 and 48 50. obesity/ or morbid obesity/ 51. pc.fs or rh.fs or th.fs. [prevention.fs. or rehabilitation.fs. or therapy.fs.] 52. 50 and 51 53. 49 or 52 Part IV: Population [adapted fromLeclercq 2013] 54. juvenile/ 55. adolescent/ 56. child/ 57. infant/ 58. baby/ 59. toddler/ 60. preschool child/ 61. school child/ 62. pediatrics/ 63. minors.tw. 64. (boy or boys or boyhood).tw. 65. girl*.tw. 66. infant*.tw. 67. (baby or babies).tw. 68. toddler?.tw. 69. (kid or kids).tw. 70. (child or childs or children* or childhood* or childcare* or schoolchild*).tw. 71. adolescen*.tw. 72. juvenil*.tw. 73. youth*.tw. 74. (teen* or preteen*).tw. 75. (underage* or under age*).tw. 76. pubescen*.tw. 77. p?ediatric*.tw. 78. or/54‐77 Part V: Part III AND IV and additional MeSH/subheading combination 79. 53 and 78 80. childhood obesity/ 81. pc.fs or rh.fs or th.fs. [prevention.fs. or rehabilitation.fs. or therapy.fs.] 82. 80 and 81 83. 79 or 82 Part VI: Study filter [Wong 2006afilter ‐ SDSSGS version] 84. random*.tw. or clinical trial*.mp. or exp treatment outcome/ Part VII: Part V + Part VI 85. 83 and 84 |
| PsycINFO (Ovid SP) |
|
Part I: Obesity 1. exp Overweight 2. (adipos* or obes*).tw. 3. (overweight* or over weight*).tw. 4. or/1‐3 Part II: Intervention 5. Weight Control/ 6. Weight Loss/ 7. Aerobic Exercise/ 8. Diets/ 9. exp Exercise/ 10. Movement Therapy/ 11. Dance Therapy/ 12. exp Physical Activity/ 13. Physical Fitness/ 14. Health Behavior/ 15. Health Promotion/ 16. Health Knowledge/ 17. Health Literacy/ 18. Health Education/ 19. Client Education/ 20. Lifestyle/ 21. Physical Education/ 22. exp Program Evaluation/ 23. Educational Programs/ 24. Educational Therapy/ 25. exp Program Development/ 26. School Based Intervention/ 27. School Counseling/ 28. Counseling/ 29. Group Counseling/ 30. Family Therapy/ 31. Support Groups/ 32. Social Support/ 33. School Counselors/ 34. exp Behavior Modification/ 35. Cognitive Behavior Therapy/ 36. Cognitive Therapy/ 37. ((obesity adj3 intervention) or program or programme or camp?).tw. 38. (lifestyle or life style).tw. 39. exercis*.tw. 40. (physic* adj (activ* or fit*)).tw. 41. (walk* or jog* or swim* or weight lift* or danc* or aerobics).tw. 42. ((physic* or strength* or resist* or circuit or weight or aerob* or cross or endurance or structur*) adj3 train*).tw. 43. (behavio?ral or behavio?r modification or psychoth* or psychosocial).tw. 44. ((group or family or cognit* or behav*) adj therap*).tw. 45. counsel?ing.tw. 46. educat*.tw. 47. ((parent? or family) adj (based or focused or directed or centered or only or led)).tw. 48. (diet* or healthy nutrition or (nutrition* adj (knowledge or educat* or therap* or program* or intervention*))).tw. 49. or/5‐48 Part III: Part I + Part II 50. 4 and 49 Part IV: Population [adapted fromLeclercq 2013] 51. minors.tw. 52. (boy or boys or boyhood).tw. 53. girl*.tw. 54. infant*.tw. 55. (baby or babies).tw. 56. toddler?.tw. 57. (kid or kids).tw. 58. (child or childs or children* or childhood* or childcare* or schoolchild*).tw. 59. adolescen*.tw. 60. juvenil*.tw. 61. youth*.tw. 62. (teen* or preteen*).tw. 63. (underage* or under age*).tw. 64. pubescen*.tw. 65. p?ediatric*.tw. 66. or/51‐65 Part V: Part III AND IV and additional MeSH/subheading combination 67. 50 and 66 Part VI: Study filter [Eady 2008filter ‐ BS version] 68. control*.tw. OR random*.tw. OR exp Treatment/ Part VII: Part V + Part VI 69. 67 and 68 |
| CINAHL (EBSCOhost) |
|
Part I: Obesity S1. MH "Obesity+" S2. TX (adipos* or obes*) S3. TX (overweight* or "over weight*") S4. S1 OR S2 OR S3 Part II: Intervention S5. MH "Weight Loss" S6. MH "Behavior Modification+" S7. MH "Counseling" S8. MH "Family Therapy" S9. MH "Support, Psychosocial" S10.MH "Support Groups" S11.MH "Program Evaluation" S12.MH "Program Implementation" S13.MH "Exercise+" S14.MH "Sports+" S15.MH "Therapeutic Exercise+" S16.MH "Physical Fitness" S17.MH "Physical Education and Training+" S18.MH "Health Education+" S19.MH "Diet+" S20.MH "Diet Therapy+" S21.MH "Health Behavior" S22.MH "Eating Behavior" S23.MH "Health Promotion" S24.MH "School Health Services+" S25.MH "Life style changes" S26.MH "Life style" S27.TX (weight N1 (reduc* or los* or control* or manage*)) S28.TX ((obesity N3 intervention) OR program OR programme OR camp#) S29.TX (lifestyle or "life style") S30.TX exercis* S31.TX (physic* N1 (activ* or fit*)) S32.TX (walk* or jog* or swim* or weight lift* or danc* or aerobics) S33.TX ((physic* or strength* or resist* or circuit or weight or aerob* or cross or endurance or structur*) N3 train*) S34.TX (behavio#ral or behavio#r modification or psychoth* or psychosocial) S35.TX ((group or family or cognit* or behav*) N1 therap*) S36.TX counsel#ing S37.TX educat* S38.TX ((parent# or family) N1 (based or focused or directed or centered or only or led)) S39.TX (diet* or "healthy nutrition" or (nutrition* N1 (knowledge or educat* or therap* or program* or intervention*))) S40.S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 Part III: Part I + Part II and additional MeSH/subheading combination S41.S4 AND S40 S42.(MH "Obesity+/DH/ED/PC/PF/RH/TH") [diet therapy or education or prevention & control or psychosocial factors or rehabilitation or therapy] S43.S41 OR S42 Part IV: Population [based onLeclercq 2013] S44.MH "Adolescence" S45.MH "Child+" S46.MH "Infant" S47.MH "Pediatrics" S48.TX minors S49.TX (boy OR boys OR boyhood) S50.TX girl* S51.TX infant* S52.TX (baby OR babies) S53.TX toddler# S54.TX (kid OR kids) S55.TX (child OR childs OR children* OR childhood* OR childcare* OR schoolchild*) S56.TX adolescen* S57.TX juvenil* S58.TX youth* S59.TX (teen* or preteen*) S60.TX (underage* or under age*) S61.TX pubescen* S62.TX (paediatric* OR paediatric*) S63.S44 OR S45 OR S46 OR S47 OR S48 OR S49 OR S50 OR S51 OR S52 OR S53 OR S54 OR S55 OR S56 OR S57 OR S58 OR S59 OR S60 OR S62 Part V: Part III AND IV and additional MeSH/subheading combination S64.S43 AND S63 S65.(MH "Pediatric Obesity/DH/ED/PC/PF/RH/TH") [diet therapy or education or prevention & control or psychosocial factors or rehabilitation or therapy] S66.S64 OR S65 Part VI: Study filter [Wong 2006bfilter ‐ SDSSGS version] S67.MH "treatment outcomes+" OR MH "experimental studies+" or random* Part VII: Part V + Part VI S68.S66 AND S67 |
| LILACS (IAHx) |
| ((((MH:"Obesity" OR MH:"Obesity, Morbid" OR MH:"Obesity, Abdominal" OR MH:"Pediatric Obesity" OR MH:"Overweight" OR adipos$ OR obes$ OR overweight$ OR "over weight" OR sobrepes$ OR "exceso de peso" OR "excesso de peso") AND (MH:"Weight Loss" OR MH:"Exercise" OR MH:"Exercise Therapy" OR MH:"Physical Education and Training" OR MH:"Exercise Movement Techniques" OR MH:"Weight Reduction Programs" OR MH:"Motor Activity" OR MH:"Behavior Therapy" OR MH:"Counseling" OR MH:"Family Therapy" OR MH:"Social Support" OR MH:"Program Evaluation" OR MH:"Diet" OR MH:"Diet Therapy" OR MH:"Patient Education as Topic" OR MH:"Health Education" OR MH:"Health Behavior" OR MH:"Health Promotion" OR MH:"Weight Reduction Programs" OR MH:"School Health Services" OR MH:"Life style" OR exerci$ OR ejerci$ OR ((physic$ OR fisic$) AND (activ$ OR ativid$ OR fit$ OR educac$ OR entrenam$ OR treinam$)) OR ((physic$ OR fisic$ OR strength$ OR forca OR fuerza OR resist$ OR circuit$ OR weight OR aerob$ OR endurance OR structur$ OR estructur$) AND train$ OR treina$ OR entrena$) OR program$ OR "estilo de vida" OR padres OR pais OR familia OR familias OR familiar OR terapia OR orienta$ OR educa$ OR diet$ OR nutric$ OR "weight reduction" OR "weight loss" OR "weight control" OR "control de peso")) OR (MH:"Obesity/diet therapy" OR MH:"Obesity, Morbid/diet therapy" OR MH:"Overweight/diet therapy" OR MH:"Obesity/prevention & control" OR MH:"Obesity, Morbid/prevention & control " OR MH:"Overweight/prevention & control" OR MH:"Obesity/rehabilitation" OR MH:"Obesity, Morbid/rehabilitation" OR MH:"Overweight/rehabilitation" OR MH:"Obesity/therapy" OR MH:"Obesity, Morbid/therapy" OR MH:"Overweight/therapy" OR MH:"Obesity/psychology" OR MH:"Obesity, Morbid/psychology" OR MH:"Overweight/psychology")) AND (MH:"Adolescent" OR MH:"Child" OR MH:"Pediatrics" OR MH:"Infant" OR minors OR boy OR boys OR girl$ OR kid OR kids OR child OR childs OR children$ OR childhood$ OR childcare$ OR schoolchild$ OR escolar$ OR adolescen$ OR preadolescen$ OR juvenil$ OR juventud$ OR youth$ OR teen$ OR preteen$ OR underage$ OR pubescen$ OR paediatri$ OR pediatri$ OR joven$ OR jovem$ OR niños OR niñas OR crianca$ OR menin$ OR "menor de edad" OR "menores de edad" OR "menor de idade" OR "menores de idade")) OR MH:"Pediatric Obesity/diet therapy" OR MH:"Pediatric Obesity/prevention & control" OR MH:"Pediatric Obesity/rehabilitation" OR MH:"Pediatric Obesity/therapy" OR MH:"Pediatric Obesity/psychology" [activated filter "Controlled Clinical Trial"] |
| ICTRP Search Portal (Advanced search) |
|
[activated "Search for clinical trials in children"]: in Title: obes* OR overweight* OR in Condition: obes* OR overweight* Recruitment Status: ALL |
| ClinicalTrials.gov (Advanced search) |
|
Conditions: obese OR overweight OR obesity Study type: Interventional Studies Age Group: Child (birth‐17) |
Appendix 2. Description of interventions
| Intervention(s) | Comparator(s) | |
| Resnicow 2015 | Parent‐only PCP motivational interviewing: 4 sessions over 2 years with the primary care provider and standard advice on healthy eating and exercise | Usual care: standard advice on healthy eating and exercise |
| Parent‐only PCP + dietician motivational interviewing: 4 sessions over 2 years with the primary care provider and 6 sessions with a dietician. Standard advice on healthy eating and exercise | ||
| Mazzeo 2014 | Parent NOURISH: parents' self efficacy to make positive changes in eating and exercise behaviours | Parent control: single session on diet and exercise and mailed out information |
| van Grieken 2013 | Parent‐only: healthy lifestyle counselling at well‐child visit and up to 3 additional sessions at 3, 6 and 12 months, included motivational interviewing; focus on targeting key lifestyle‐related behaviours | Usual care control: general information about healthy lifestyle |
| Small 2013 | Parent‐only: focus on healthy habits in young children, nutritional information, information regarding increasing physical activity | Parent control: provision of educational age‐appropriate, evidence‐based health and safety information and group sessions |
| Esfarjani 2013 | Parent‐only: educational sessions on nutrition, physical activity and behavioural control | Parent control: 2 parent training sessions |
| Moens 2012 | Parent‐only: 5‐month intervention, 6 group meetings of 2 hours, focused on education of dietary requirements and training for parenting behavioural skills | Waiting list control: for 6 months |
| Raynor 2012a | Parent‐only: included behavioural strategies focused on increasing child growth monitoring and providing feedback to families | (I2) C1: parent intervention diet 'decrease': parent intervention as in parent‐only intervention and children and parents intervention focusing on decreasing sugar‐sweetened beverage and sweet and salty snack food intake |
| (I3) C2: parent intervention diet 'increase': parent intervention as in parent‐only intervention and children and parents intervention focusing on increasing fruit, vegetable and low‐fat dairy intake | ||
| Raynor 2012b | Parent‐only: included behavioural strategies focused on increasing child growth monitoring and providing feedback to families | (I2) C1: parent intervention diet and activity 'traditional': parent intervention as in parent‐only intervention and children and parents intervention focusing on decreasing sugar‐sweetened beverage intake and increasing physical activity |
| (I3) C2: parent intervention diet and activity 'substitute': parent intervention as in parent‐only intervention and children and parents intervention focusing on increasing low‐fat milk intake and decreasing TV watching | ||
| Magarey 2011 | Parent healthy lifestyle group: delivered in 4 sessions, standardised and evaluated generic parenting programme widely used in Australia and provides comprehensive facilitator training; followed by 8 healthy lifestyle sessions (as described for healthy lifestyle group) | Healthy lifestyle group: delivered in 8 sessions, included recommendations on specific core food servings; practical skills for healthy eating, reduced sedentary behaviours and increased activity; and monitoring of lifestyle behaviours and roles and responsibilities around eating, managing appetite, self esteem and teasing |
| Jansen 2011 | Parental CBT: included a focus on behavioural and nutritional components | Waiting list control: 6 month no treatment group |
| Collins 2011 | Parent‐only diet: includes behavioural techniques for food choices |
C1: parent‐child (physical activity): includes physical activity goals and key skills C2: parent‐child (physical activity and diet): incorporates both the physical activity and diet‐only components of the other interventions |
| Boutelle 2011 | Parent‐only behavioural change: included self monitoring of targeted behaviours, positive reinforcement, stimulus control, pre‐planning and modelling | Parent‐child behavioural: included self monitoring of targeted behaviours, positive reinforcement, stimulus control, pre‐planning and modelling for parents and material taught in the child groups was similar but presented in an age‐appropriate manner |
| West 2010 | Parent‐only behavioural change, 'Group Lifestyle Triple P': included positive‐parenting strategies, physical activity strategies and nutrition strategies | Waiting list control: included a physical activity and nutritional advice components |
| Resnick 2009 | Educational material plus personal encounters: included choices of topics such as biological, social and environmental influences on childhood overweight; nutrition advice, physical activity guidelines | Educational material: included components on increased physical activity and nutritional components |
| Estabrooks 2009 | Parent group and IVR counselling: 2 group sessions addressing behavioural health skills and knowledge of weight, nutrition and physical activity. Based on social‐ecological theory. Utilised the workbook in the control group and followed by 10 IVR sessions including prompts for physical activity, nutrition, behavioural components | Group sessions: 2 group sessions as per group + IVR intervention; utilised the workbook |
| Control: workbook group: 61‐page workbook to promote physical activity, healthy habits, nutrition | ||
| Munsch 2008 | Mother‐only CBT: included nutrition and eating behaviour, physical activity, social competences, body concept, relapse prevention; children attended a relaxation training session | Mother‐child CBT: included the same components as the mother‐only intervention; children received sessions on nutrition and eating behaviour, basic nutritional education, reinforcement and tokens, lessons in physical activity, social competencies, developing a positive body concept, relapse prevention |
| Janicke 2008 | Parent‐only: includes behavioural, nutritional and physical activity components | C1: parent‐child: includes behavioural, nutritional and physical activity components |
| C2: waiting list control: no active treatment | ||
| Golley 2007 | Parenting‐skills training with intensive lifestyle education: behavioural change intervention including nutritional components; also 7 intensive lifestyle support group sessions | C1: parenting‐skills training: behavioural change intervention including nutritional components |
| C2: waiting list control: general healthy‐lifestyle pamphlet | ||
| Golan 2006 | Parent‐only: included behavioural, nutritional and physical activity components | Parent‐child: similar to the parent‐only but including activities for the children |
| Aragona 1975 | I1: parent‐only response‐cost plus reinforcement: nutritional information, exercise instructions, weight and calorie information, behavioural techniques including reinforcement techniques | Waiting list control |
| I2: parent‐only ‐ response‐cost: as above without the reinforcement techniques | ||
| I: intervention; C: comparator; CBT: cognitive behavioural therapy; IVR: interactive voice response; NOURISH: Nourishing Our Understanding of Role modelling to Improve Support and Health; PCP: primary care providers; PHL: parent healthy lifestyle; TV: television | ||
Appendix 3. Baseline characteristics (I)
| Intervention(s) and comparator(s) | Duration of intervention (duration of follow‐up) | Description of participants | Study period (year to year) | Country | Setting | Ethnic groups (%) | Socioeconomic status (%) | |
| Resnicow 2015 | I1: parent‐only PCP motivational interviewing | 2 years (follow‐up: 2 years) | Parents of children aged 2‐8 years with BMI ≥ 85th and ≤ 97th percentile | ‐ | USA | Primary care | White: 53.6 Black: 11.0 Hispanic: 30.14 Asian: 1.14 Other: 3.83 |
Income < USD 40,000: 38.6%; ≥ USD 40,000: 61.4% Education < college: 70.1%; ≥ college: 29.9% |
| I2: parent‐only PCP + dietician motivational interviewing | White: 59.1 Black: 6.09 Hispanic: 20.9 Asian: 8.7 Other: 5.2 |
Income < USD 40,000: 29.8%; ≥ USD 40,000; 70.2% Education < college: 52.6%; ≥ college: 47.4% |
||||||
| C: usual care | White: 67.9 Black: 2.6 Hispanic: 13.3 Asian: 6.6 Other: 9.7 |
Income < USD 40,000: 27.2%; ≥ USD 40,000: 72.8% Education < college: 61.8%; ≥ college: 38.2% |
||||||
| Mazzeo 2014 | I: parent NOURISH | 12 weeks (24 weeks) | Parents of overweight children aged 6‐11 years | 2008‐2009 | USA | Community | ‐ | ‐ |
| C: parent control | ||||||||
| van Grieken 2013 | I: parent‐only | up to 12 months (at 24 months) | Parents of overweight or obese children aged 5 years | September 2007‐October 2008 | The Netherlands | Community (youth health care centres) | Dutch: 75.8 | Mother's education level low/mid: 34.8; mid‐high/high: 65.2 |
| C: usual care | Dutch: 80.6 | Mother's education level low/mid: 31.5; mid‐high/high: 68.5 | ||||||
| Small 2013 | I: parent‐only | 16‐24 weeks (40‐48 weeks) | Parents of overweight or obese children aged 4‐8 years | ‐ | USA | Primary care office | White: 63 Black: 9 Hispanic: 24 Other: 4 | Mothers' education:
High school degree or less: 24.3
At least some college: 75.7 Mothers' marital status: Married: 75.7 Single/divorced/widowed: 24.3 Family structure: 2 parent: 97 1 parent: 3 |
| C: control | White: 66 Hispanic: 25 Other: 7 |
Mothers' education:
High school degree or less: 29.7
At least some college: 70.3 Mothers' marital status: Married: 74.1 Single/divorced/widowed: 25.9 Family structure: 2 parent: 81.5 1 parent: 18.5 |
||||||
| Esfarjani 2013 | I: parent‐only | 6 months (follow‐up unclear) | Parents of obese children aged 7 years | ‐ | Iran | ‐ | ‐ | Father's education Under high school diploma: 4.3 High school diploma: 41.4 Higher than high school diploma: 54.3 |
| C: control | Father's education Under high school diploma: 7 High school diploma: 38.4 Higher than high school diploma: 54.7 | |||||||
| Moens 2012 | I: parent‐only | 6 months (6 months) | Parents of overweight or obese children aged 6‐12 years | 2001‐2006 | Belgium | University research setting | 100% European (Caucasian) | Index of social position, low 26%, middle 52%, high 22% |
| C: waiting list control | 100% European (Caucasian) | Index of social position, low 0%, middle 75%, high 25% | ||||||
| Raynor 2012a | I: parent‐only | 24 weeks (12 months) | Parents of overweight or obese children | November 2005‐September 2007 | USA | University and primary care | White: 90.9 Hispanic: 21.2 | ‐ |
| C1: parent ‐ diet decrease | White: 80 Hispanic: 20 | |||||||
| C2: parent ‐ diet increase | White: 87 Hispanic: 15.2 | |||||||
| Raynor 2012b | I: parent‐only | 24 weeks (12 months) | Parents of overweight or obese children | November 2005‐September 2007 | USA | University and primary care | White: 93.1 Hispanic: 13.8 | ‐ |
| C1: parent ‐ diet and activity traditional | White: 84.6 Hispanic: 11.5 | |||||||
| C2: parent ‐ diet and activity substitute | White: 92.3 Hispanic: 7.7 | |||||||
| Magarey 2011 | I: parent healthy lifestyle | 24 weeks (104 weeks) | Parents of children classified as overweight aged 5‐9 years | May 2004‐estimated May 2006 | Australia | Outpatient clinic | ‐ | Reported for area of residence (Sydney or Adelaide) only, not by study arm |
| C: healthy lifestyle | ||||||||
| Jansen 2011 | I: parent CBT | 12 weeks (24 weeks) | Parents of overweight children aged 7‐13 years | ‐ | Netherlands | Community health centre, university site | ‐ | ‐ |
| C: waiting list control | ||||||||
| Collins 2011 | I: parent‐only diet | 24 weeks (104 weeks) | Parents of overweight children aged 5‐9 years | January 2005‐2008 | Australia | Outpatient clinic | ‐ | ‐ |
| C1: parent‐child (physical activity) | ||||||||
| C2: parent‐child (physical activity + diet) | ||||||||
| Boutelle 2011 | I: parent‐only | 20 weeks (44 weeks) | Parents and their overweight or obese (> 85th BMI percentile) children aged 8‐12 years | ‐ | USA | University | ‐ | Total household income: < USD 20,000: 2.6% USD 20,001‐40,000: 7.9% USD 40,001‐60,000: 18.4% > $60,000: 63.2% Don't know: 7.9% Education‐highest parent: Less than high school 0.0% High school 7.9% Vocational school 7.9% Some college 23.7% College graduate 42.1% Advanced degree 18.4% |
| C: parent‐child | Total household income: < USD 20,000: 5.0% USD 20,001‐40,000: 10.0% USD 40,001‐60,000: 15.0% > USD 60,000: 65.0% Don't know: 5.0% Education‐highest parent: Less than high school 2.5% High school 12.5% Vocational school 5.0% Some college 25.0% College graduate 35.0% Advanced degree 20.0 | |||||||
| West 2010 | I: parent‐only | 12 weeks (1 year) | Children described as overweight by parents, aged 4‐11 years, and their parents | September 2003‐October 2004 | Australia | University, hospitals and schools | White: 88.5 Mediterranean: 3.8 Asian: 3.8 Indigenous: 3.8 | Mother's education: secondary only: 25.0; tertiary: 75.0 Father's education: secondary only: 20.9; tertiary: 79.1 Mother employed: no: 38.5; yes: 61.5 Father employed: no: 7.0; yes: 93.0 Annual family income: < AUD 20,000: 3.8; AUD 20,000‐40,000: 25.0; AUD 40,000‐100,000: 51.9; > AUD 100,000: 19.2 Mean (SD): mother's paid work (hour/week): 10.50; father's paid work (hour/week): 6.83 |
| C: control | White: 85.7 Mediterranean: 8.2 Asian: 4.1 Indigenous: 2.0 | Mother's education:
secondary only: 34.7;
tertiary: 65.3
Father's education:
secondary only: 25.6;
tertiary: 74.4 Mother employed: no: 32.7; yes: 67.3 Father employed: no: 5.1; yes: 94.9 Annual family income: < AUD 20,000: 0; AUD 20,000‐40,000: 22.4; AUD 40,000‐100,000: 51.0; > AUD 100,000: 26.5 Mean (SD): mother's paid work (hour/week): 12.33; father's paid work (hour/week): 11.91 |
||||||
| Resnick 2009 | I: educational material + personal encounters | 18 weeks (unclear follow‐up, 41 week mail out) | Parents of overweight children aged 5‐11 years | October 2006‐ April 2007 | USA | Community | ‐ | ‐ |
| C: educational material | ||||||||
| Estabrooks 2009 | I: parent group + IVR | 12‐24 weeks (52 weeks) | Parents of children aged 8‐12 years with a BMI > 85th percentile for their age | May 2004‐December 2007 | USA | Outpatient clinic | White: 60 Hispanic: 30 |
‐ |
| I2: parent group | White: 69 Hispanic: 19 |
|||||||
| C: parent workbook | White: 59 Hispanic: 29 |
|||||||
| Munsch 2008 | I: mother only | 10 weeks (34 weeks) | Mothers of children aged 8‐12 years with a BMI > 85th percentile adjusted for age and gender | ‐ | Switzerland | Efficacy of US Pediatric obesity primary care guidelines: 2 randomised trials Outpatient clinic and university |
‐ | ‐ |
| C: mother‐child | ||||||||
| Janicke 2008 | I: parent‐only | 16 weeks (40 weeks) | Parents of overweight children aged 8‐13 years | ‐ | USA | Community | Child: White: 80.8 African‐American: 3.8 Hispanic: 3.8 Bi‐racial: 11.5 | Annual family income: ≤ USD 19,999: 19.2% USD 20,000‐59,999: 46.1% ≥ USD 60,000: 34.6% |
| C1 parent‐child | Child: White: 66.7 African‐American: 12.5 Hispanic: 16.7 Bi‐racial: 4.2 | Annual family income: ≤ USD 19,999: 16.6% USD 20,000‐59,999: 45.8% ≥ USD 60,000: 29.2% | ||||||
| C2: waiting list control | Child: White 80.9 African‐American 14.3 Hispanic 4.8 Bi‐racial 0 | Annual family income: ≤ USD 19,999: 19.1% USD 20,000‐59,999: 61.9% ≥ USD 60,000: 19.1% | ||||||
| Golley 2007 | I: parent + lifestyle education | 24 weeks (48 weeks) | Parents of overweight children aged 6‐9 years | July 2002‐August 2003 | Australia | Outpatient clinic | ‐ | ‐ |
| C1 parent | ||||||||
| C2: waiting list control | ||||||||
| Golan 2006 | I: parent‐only | 26 weeks (18 months) | Parents of overweight children aged 6‐11 years | ‐ | Israel | Unclear | ‐ | ‐ |
| C: parent‐child | ||||||||
| Aragona 1975 | I1: parent‐only + reinforcement | 12 weeks (51 weeks) | Parents of overweight girls aged 5‐10 years | ‐ | USA | ‐ | ‐ | ‐ |
| I2: parent‐only | ||||||||
| C: control | ||||||||
| "‐" denotes not reported C: comparator; CBT: cognitive behavioural therapy; I: intervention; IVR: interactive voice response; NOURISH: Nourishing Our Understanding of Role modelling to Improve Support and Health; PCP: primary care providers; SD: standard deviation | ||||||||
Appendix 4. Baseline characteristics (II)
| Intervention(s) and comparator(s) | Sex [female %]a | Age [mean (SD)] | BMI measures [mean kg/m2 (SD)] | Body weight [mean kg (SD)] |
Parental weight [mean BMI (SD) or as stated] |
Comedications/cointerventions/ comorbidities | |
| Resnicow 2015 | I1: parent‐only PCP motivational interviewing | 57 | 5.1 (1.9) | Percentile: 92.2 (3.3) | ‐ | BMI: 30.1 (7.4) | ‐ |
| I2: parent‐only PCP + dietician motivational interviewing | 60 | 5.3 (1.8) | Percentile: 92.1 (3.4) | ‐ | BMI: 28.5 (6.4) | ||
| C: usual care | 53 | 4.9 (1.7) | Percentile: 91.5 (3.3) | ‐ | BMI: 28.4 (6.8) | ||
| Mazzeo 2014 | I: parent NOURISH | 68 | ‐ | BMI percentile: 98.47 (2.24) | ‐ | BMI: 34.2 (9.3) | ‐ |
| C: parent control | 64 | ‐ | BMI percentile: 97.86 (2.67) | ‐ | |||
| Van Grieken 2013 | I: parent‐only | 61 | 5.72 (0.42) | BMI: 18.16 (0.63) BMI SDS: 1.93 (0.38) |
‐ | Normal: 55.5% Overweight/obese: 44.5% |
‐ |
| C: usual care | 63 | 5.8 (0.45) | BMI: 18.10 (0.61) BMI SDS: 1.88 (0.35) |
‐ | Normal: 56.6% Overweight/obese: 43.4% |
||
| Small 2013 | I: parent | 52 | 5.73 (1.38) | BMI: 21.93 (3.51) BMI percentile: 96.7 (4.04) |
32.71 (10.14) | BMI: 31.56 (8.80) | ‐ |
| C: control | 70 | 5.41 (1.5) | BMI: 20.36 (2.71) BMI percentile: 95.4 (4.62) |
28.25 (8.08) | BMI: 31.89 (8.79) | ||
| Esfarjani 2013 | I: parent‐only | ‐ | 7 | 22.7 (2.2) | 36.4 (4.8) | ‐ | ‐ |
| C: control | ‐ | 7 | 22.5 (1.9) | 36.6 (4.7) | ‐ | ||
| Moens 2012 | I: parent‐only | 65 | 9.10 (1.35) | BMI %: 147.5 (17.93) | ‐ | Mother: 26.92 (5.43) Father: 27.14 (4.16) |
‐ |
| C: waiting list control | 58 | 9.26 (1.45) | BMI %: 140.45 (10.15) | ‐ | Mother: 24.75 (3.02) Father: 28.16 (3.15) |
||
| Raynor 2012a | I: parent‐only | 61 | 6.8 (1.8) | BMI: 34.6 ± 9.7 | ‐ | ‐ | ‐ |
| C1: parent ‐ diet decrease | 63 | 7.2 (1.6) | BMI: 33.4 ± 8.3 | ‐ | ‐ | ||
| C2: parent ‐ diet increase | 61 | 7.6 (1.6) | BMI: 32.2 ± 7.2 | ‐ | ‐ | ||
| Raynor 2012b | I: parent‐only | 59 | 6.7 (1.6) | BMI: 33.2 ± 9.1 | ‐ | ‐ | ‐ |
| C1: parent ‐ diet + activity traditional | 65 | 7.2 (1.5) | BMI: 30.5 ± 7.2 | ‐ | ‐ | ||
| C2: parent ‐ diet + activity substitute | 58 | 7.4 (1.3) | BMI: 33.6 ± 8.5 | ‐ | ‐ | ||
| Margarey 2011 | I: parent healthy lifestyle | 56 | All: 8.2 (1.2) | BMI (all): 24.1 (3.22) BMI z score: 2.77 (0.58) |
All: 44.4 (9.82) | ‐ | ‐ |
| C: healthy lifestyle | All: 56 | All: 8.2 (1.2) | BMI (all): 24.1 (3.22) BMI z score 2.68 (0.65) |
All: 44.4 (9.82) | ‐ | ||
| Jansen 2011 | I: parent CBT | ‐ | ‐ | BMI percentile: 96.8 (2.93) | ‐ | BMI: 28.30 (4.53) | ‐ |
| C: waiting list control | ‐ | ‐ | BMI percentile: 95.9 (3.38) | ‐ | BMI: 29.35 (6.33) | ||
| Collins 2011 | I: parent‐only diet | 62 | 8.2 (1.2) | BMI: 24.6 (3.0) BMI z score: 2.8 (0.6) | 46.3 (8.6) | ‐ | ‐ |
| C1: parent‐child (physical activity) | 60 | 8.3 (1.0) | BMI: 25.2 (4.1) BMI z score: 2.8 (0.7) | 48 (10.8) | ‐ | ‐ | |
| C2: parent‐child (physical activity + diet) | 55 | 8.1 (1.2) | BMI: 24.4 (3.7) BMI z score: 2.8 (0.7) | 45.5 (12.2) | ‐ | ‐ | |
| Boutelle 2011 | I: parent‐only | 50 | 10.81 (1.31) | Child BMI percentile: 98.37 (1.85) Child BMI z score: 2.29 (0.38) Child BMI: 30.48 (6.08) |
‐ | Parent BMI: 32.47 (8.25) n = 39 |
‐ |
| C: parent‐child | 70 | 10.08 (1.15) | Child BMI percentile: 98.34 (1.37) Child BMI z score: 2.25 (0.34) Child BMI: 28.26 (4.64) |
‐ | Parent BMI: 31.47 (7.46) n = 40 |
||
| West 2010 | I: parent‐only | 69 | 8.58 (1.69) | BMI z score: 2.15 (0.43) | ‐ | Parent BMI range (n, %) Healthy weight: 20 (38.5) Overweight: 11 (21.2) Obese: 21 (40.4) | ‐ |
| C: control | 65 | 8.5 (1.65) | BMI z score: 2.11 (0.46) | ‐ | Parent BMI range (n, %) Healthy weight: 18 (36.7) Overweight: 12 (24.5) Obese: 19 (38.8) | ||
| Resnick 2009 | I: educational material + personal encounters | ‐ | ‐ | BMI percentile: 94.1 (4.3) | ‐ | BMI: 25.6 | ‐ |
| C: educational material | ‐ | ‐ | BMI percentile: 94.1 (4.4) | ‐ | BMI: 26.2 | ||
| Estabrooks 2009 | I: parent group + IVR | 41 | 10.7 | BMI: 27.1 BMI z score: 2.04 |
‐ | ‐ | ‐ |
| I2: parent group | 42 | 10.6 | BMI: 27.4 BMI z score: 2.07 |
‐ | ‐ | ||
| C: parent workbook | 61 | 11 | BMI: 27.1 BMI z score: 2.00 |
‐ | ‐ | ||
| Munsch 2008 | I: mother‐only | 63 | 10.6 (1.5) | BMI: 28.0 (5.4), n = 21 BMI SDS: 2.61 |
‐ | BMI: 26.9 (3.9) | ‐ |
| C: mother‐child | 59 | 10.3 (1.4) | BMI: 26.5 (3.3) BMI SDS: 2.40 |
‐ | BMI: 29.6 (7.5) | ||
| Janicke 2008 | I: parent‐only | 46 | 11.5 (1.3) | BMI z score: 2.16 (0.35) | ‐ | BMI: 35.47 (8.2) | ‐ |
| C1 parent‐child | 63 | 11.03 (1.6) | BMI z score: 2.13 (0.43) | ‐ | BMI: 32.86 (6.8) | ||
| C2: waiting list control | 76 | 11.02 (1.81) | BMI z score: 2.02 (0.41) | ‐ | BMI: 35.66 (9.3) | ||
| Golley 2007 | I: parent + lifestyle education | 63 | ‐ | BMI z score: 2.74 (0.58) | ‐ | ‐ | ‐ |
| C1 parent | 65 | ‐ | BMI z score: 2.76 (0.58) | ‐ | ‐ | ||
| C2: waiting list control | 64 | ‐ | BMI z score: 2.75 (0.39) | ‐ | ‐ | ||
| Golan 2006 | I: parent‐only | 59 | 8.75 (1.9) | 24.2 (3.0) | 47.1 (12.4) | Weight: kg Mothers: 72.7 (11.1) Fathers: 100.9 (24.7) |
‐ |
| C: parent‐child | 50 | 8.7 (2.0) | 24.3 (3.6) | 45.5 (15.9) | Weight: kg Mothers: 79.1 (15.5) Fathers: 102.3 (19.1) |
||
| Aragona 1975 | I1: parent‐only + reinforcement | 100 | 9.4 | BMIb: 21.72 (2.03) | 47.89 (7.76) | ‐ | ‐ |
| I2: parent‐only | 100 | 10 | BMIb: 22.76 (1.58) | 47.42 (5.70) | ‐ | ||
| C: control | 100 | 8.3 | BMIb: 23.06 (3.04) | 45.04 (14.51) | ‐ | ||
| '‐' denotes not reported aSex of the overweight child bCalculated by review authors BMI: body mass index; BMI SDS: standard deviation of BMI: C: comparator; CBT: cognitive behavioural therapy; I: intervention; IVR: interactive voice response; n: number of participants; NOURISH: Nourishing Our Understanding of Role modelling to Improve Support and Health; PCP: primary care providers; SD: standard deviation; TV: television | |||||||
Appendix 5. Matrix of study endpoints (publications and trial documents)
| Endpoints quoted in trial document(s) (ClinicalTrials.gov, FDA/EMA document, manufacturer's website, published design paper)a | Study results/ publications available in trials register | Endpoints quoted in publication(s)b,c | Endpoints quoted in abstract of publication(s)b,c | |
| Resnicow 2015 |
Source: NCT01335308 Primary outcome measure(s): BMI percentile |
No (last verified: March 2011) History of changes: 0 documented changes |
Primary outcome measure(s): BMI percentile | Primary outcome measure(s): BMI percentile |
| Secondary outcome measure(s): fruit + vegetable consumption, sweetened beverage consumption, change in physical activity | Secondary outcome measure(s): ‐ | Secondary outcome measure(s): ‐ | ||
| Other outcome measure(s): ‐ | Other outcome measure(s): ‐ | Other outcome measure(s): ‐ | ||
| Mazzeo 2014 |
Source: NCT00628030 Primary outcome measure(s):
|
Yes (last verified: July 2015) History of changes: 7 documented changes |
Primary outcome measure(s): BMI | Primary outcome measure(s): child BMI |
Secondary outcome measure(s):
|
Secondary outcome measure(s): Three Factor Eating Questionnaire; Child Feeding Questionnaire; dietary intake (Block Food Screener); Pediatric Health‐Related Quality of Life; Pubertal status (self report) | Secondary outcome measure(s): parents satisfaction, parent behaviour change | ||
| Other outcome measure(s): ‐ | Other outcome measure(s): ‐ | Other outcome measure(s): ‐ | ||
| van Grieken 2013 |
Source: ISRCTN04965410, NTR921 Primary outcome measure(s): BMI, waist circumference |
No (last verified: July 2015) History of changes: no documented changes |
Primary outcome measure(s): BMI, waist circumference | Primary outcome measure(s): BMI |
| Secondary outcome measure(s): levels of the 4 target overweight‐reducing and overweight‐inducing behaviours, i.e. being physically active, eating breakfast daily, drinking sweet beverages and watching TV or playing on a computer; health‐related quality of life; attitudes of parents regarding the 4 target behaviours; absence or presence of indicators of negative adverse effects, i.e. worry, stigmatisation, lowered self esteem and development of relative underweight | Secondary outcome measure(s): height, overweight prevalence, process evaluation, child‐health behaviours (breakfast, sweet beverages, playing outside and TV viewing) | Secondary outcome measure(s): minutes of outside play or TV viewing, having breakfast, number drinks of sweet beverages | ||
| Other outcome measure(s): ‐ | Other outcome measure(s): ‐ | Other outcome measure(s): ‐ | ||
| Small 2013 | Source: N/T | Primary outcome measure(s): ‐ | Primary outcome measure(s): ‐ | |
| Secondary outcome measure(s): ‐ | Secondary outcome measure(s): ‐ | |||
| Outcomes not specified as primary or secondary: BMI percentile; waist circumference; waist for height ratio | Other outcome measure(s): waist circumference, waist‐by‐height ratio, BMI and BMI percentile | |||
| Esfarjani 2013 | Source: N/T | Primary outcome measure(s): ‐ | Primary outcome measure(s): ‐ | |
| Secondary outcome measure(s): ‐ | Secondary outcome measure(s): ‐ | |||
| Outcomes not reported as primary or secondary: height, weight, BMI, waist circumference, hip circumference, fasting blood glucose (data not extracted), triglyceride (data not extracted), total cholesterol (data not extracted), HDL‐cholesterol (data not extracted, LDL cholesterol (data not extracted), food consumption (data not extracted), watching TV (data not extracted), playing on computer (data not extracted), walking time (data not extracted) | Other outcome measure(s): weight, waist and hip circumference, cholesterol, serum triglycerides, food group consumption, TV and computer time, walking time | |||
| Moens 2012 | Source: N/T | Primary outcome measure(s): see below | Primary outcome measure(s): ‐ | |
| Secondary outcome measure(s): see below | Secondary outcome measure(s): ‐ | |||
| Outcomes not reported as primary or secondary: height, weight, Dutch eating behaviour questionnaire (child and parent versions); Ghent Parental Behaviour Scale; health principles questionnaire, Hollingshead Index of Social Position | Other outcome measure(s): BMI, parental report of child's eating behaviour, familial health principles | |||
| Raynor 2012b |
Source: NCT00259324 Primary outcome measure(s): BMI z score |
No (last verified: July 2015) History of changes: 6 documented changes |
Primary outcome measure(s): ‐ | Primary outcome measure(s): ‐ |
| Secondary outcome measure(s): eating and activity behaviours | Secondary outcome measure(s): ‐ | Secondary outcome measure(s): ‐ | ||
| Other outcome measure(s): | Outcomes not specified as primary or secondary: weight, height, BMI and BMI z score; dietary intake (food diaries); leisure time activity (Previous Day Physical Activity Recall) | Other outcome measure(s): BMI z score, energy intake | ||
| Raynor 2012a |
Source: NCT00200265 Primary outcome measure(s): BMI z score |
No (last verified: July 2015) History of changes: 6 documented changes |
Primary outcome measure(s): ‐ | Primary outcome measure(s): ‐ |
| Secondary outcome measure(s): eating and activity behaviours | Secondary outcome measure(s): ‐ | Secondary outcome measure(s): ‐ | ||
| Other outcome measure(s): ‐ | Outcomes not specified as primary or secondary: weight, height, BMI and BMI z score; dietary intake (food diaries); leisure time activity (Previous Day Physical Activity Recall) | Other outcome measure(s): BMI z score, energy intake | ||
| Magarey 2011 |
Source: ACTRN12606000120572 Primary outcome measure(s): BMI z score |
No (last verified: February 2013) History of changes: no documented changes |
Primary outcome measure(s): BMI z score | Primary outcome measure(s): BMI z score |
| Secondary outcome measure(s): waist circumference, fasting lipids, triglyceride, insulin, and glucose, blood pressure, health‐related quality of life, body satisfaction, parenting, parental BMI, eating and activity behaviours, health belief, programme evaluation | Secondary outcome measure(s): Program Impact (Parenting Sense of Competence Scale); Parenting (Alabama Parenting questionnaire); health‐related quality of life, waist circumference, fasting lipids, triglycerides, insulin, glucose, blood pressure, body satisfaction, eating and activity behaviours, health belief, programme satisfaction | Secondary outcome measure(s): waist z score | ||
| Other outcome measure(s): ‐ | Other outcome measure(s): ‐ | Other outcome measure(s): ‐ | ||
| Jansen 2011 | Source: N/T | Primary outcome measure(s): ‐ | Primary outcome measure(s): ‐ | |
| Secondary outcome measure(s): ‐ | Secondary outcome measure(s): ‐ | |||
| Outcomes not specified as primary or secondary: weight; height; BMI percentile, eating psychopathology (Child Eating Disorders Examination Questionnaire), eating behaviours (self report), physical activity (Baecke Questionnaire), self esteem (Self‐Perception Profile for Children), negative thoughts (Heavy Thoughts Questionnaire), knowledge test, motivation (therapist rated) | Other outcome measure(s): BMI percentile, relapse, psychopathology, self esteem and negative thoughts | |||
| Collins 2011 |
Source: NCT00107692 Primary outcome measure(s): ‐ |
No (last verified: September 2006) History of changes: 5 documented changes |
Primary outcome measure(s): BMI z score and waist circumference | Primary outcome measure(s): BMI z score, waist measurements |
| Secondary outcome measure(s): ‐ | Secondary outcome measure(s): blood pressure, cholesterol, C‐reactive protein, triglycerides, glucose, insulin, energy intake, physical activity, movement and skill proficiency, perceived athletic competence, screen behaviours | Secondary outcome measure(s): metabolic outcomes | ||
| Outcomes not stated as primary or secondary: BMI SD score, height, weight, and waist circumference; metabolic profile measures: blood pressure; cholesterol, triglycerides; glucose and insulin; physical activity energy expenditure and sedentary activities: total kilocalories expended and time spent in sedentary activities; dietary energy intake: 4‐day weighed food record (2 week days and the weekend), parent selection of lower fat items in the household grocery shopping and behaviour changes related to a healthy lifestyle; actual and perceived competence: Test of Gross Motor Development and the Self‐Perception Profile for Children. Activity of daily living: Sit‐to‐stand transfer | Other outcome measure(s): ‐ | Other outcome measure(s): ‐ | ||
| Boutelle 2011 | Source: N/T | Primary outcome measure(s): weight, BMI (basis of non‐inferiority test) | Primary outcome measure(s): inferiority of treatment group on child weight loss | |
| Secondary outcome measure(s): dietary intake (Block Kids questionnaire), physical activity (Physical Activity Questionnaire for Older Children), BMI percentile, BMI z score | Secondary outcome measure(s): parent weight loss and child physical activity, caloric intake | |||
| Other outcome measure(s): ‐ | Other outcome measure(s): ‐ | |||
| West 2010 | Source: N/T | Primary outcome measure(s): BMI z score | Primary outcome measure(s): child BMI z score | |
| Secondary outcome measure(s): weight‐related problem behaviour and parenting self efficacy (Lifestyle Behaviour Checklist); ineffective parenting (Parenting Scale) | Secondary outcome measure(s): weight‐related problem behaviour, confidence in managing children's weight‐related behaviour | |||
| Other outcome measure(s): ‐ | Other outcome measure(s): ‐ | |||
| Resnick 2009 | Source: N/T | Primary outcome measure(s): ‐ | Primary outcome measure(s): ‐ | |
| Secondary outcome measure(s): ‐ | Secondary outcome measure(s): ‐ | |||
| Outcomes not specified as primary or secondary: sources of information, parent confidence, behavioural questions, patient satisfaction (all self report measures), BMI | Other outcome measure(s): BMI | |||
| Estabrooks 2009 |
Source: NCT00433901 Primary outcome measure(s): child BMI |
No (last verified: July 2010) History of changes: 2 documented changes |
Primary outcome measure(s): BMI z score | Primary outcome measure(s): child BMI z scores |
| Secondary outcome measure(s): parent BMI, objective measure of physical activity using child accelerometer, Kids Eating Disorder Survey (KEDS), Peds QOL survey, SE (self efficacy), self report of physical activity, self report of sedentary activity, children's block food frequency survey, parent's home environment survey, parent self efficacy, parent demographics, parent quality of life, parent rapid assessment of physical activity, parent fat and fibre survey, parent health literacy, child/family medical history, economic survey type of medical weight management services | Secondary outcome measure(s): physical activity and sedentary behaviour (Youth Behavioural Risk Survey question); fruit, vegetable and sugared‐drink consumption (Block Kids Questionnaire); eating disorder symptoms (Kids Eating Disorders Survey); health‐related quality of life (Peds QOL survey), self efficacy | Secondary outcome measure(s): symptoms of eating disorders and body image | ||
| Other outcome measure(s): ‐ | Other outcome measure(s): ‐ | Other outcome measure(s): ‐ | ||
| Munsch 2008 | Source: N/T | Primary outcome measure(s): ‐ | Primary outcome measure(s): ‐ | |
| Secondary outcome measure(s): ‐ | Secondary outcome measure(s): ‐ | |||
| Outcomes not specified as primary or secondary: per cent overweight; depression (Depressionsinventar für Kinder und Jugendliche); anxiety (State‐Trait Anxiety Inventory for children) Social Anxiety Scale for Children‐Revised); behaviour problems (Child Behaviour CheckList), mental disorders (Diagnostisches Interview bei psychischen Störungen im Kindes‐ und Jugendalter (K‐DIPS)), binge eating (screening interview) | Other outcome measure(s): % overweight, general behaviour problems (externalising and internalising behaviour problems), global and social anxiety, and depression | |||
| Janicke 2008 | Source: N/T | Primary outcome measure(s): BMI z score (basis of power calculation) | Primary outcome measure(s): BMI z score, | |
| Secondary outcome measure(s): Youth/Adolescent Food Frequency Questionnaire | Secondary outcome measure(s): self esteem, cost | |||
| Other outcome measure(s): ‐ | Other outcome measure(s): ‐ | |||
| Golley 2007 |
Source: ACTRN12606000119594 Primary outcome measure(s): BMI z score |
No (last verified: February 2013) History of changes: no documented changes |
Primary outcome measure(s): BMI z score | Primary outcome measure(s): BMI z score |
| Secondary outcome measure(s): waist circumference, fasting lipids, triglyceride, insulin, and glucose, blood pressure, health‐related quality of life, body satisfaction, parental weight status and waist circumference, eating and activity behaviours, parental competency, parental satisfaction | Secondary outcome measure(s): waist circumference, blood pressure; fasting glucose; total cholesterol; high‐density lipoprotein cholesterol; low‐density lipoprotein cholesterol; triacylglycerol; programme evaluation; satisfaction; health related quality of life | Secondary outcome measure(s): waist circumference z score | ||
| Other outcome measure(s): ‐ | Other outcome measure(s): ‐ | Other outcome measure(s): ‐ | ||
| Golan 2006 | Source: N/T | Primary outcome measure(s): weight loss (basis of power calculation) | Primary outcome measure(s): % overweight at end of programme (6 months) and 1‐year follow‐up | |
| Secondary outcome measure(s): weight; height, BMI z score; family eating questionnaire; parenting style (Parental Authority Questionnaire) | Secondary outcome measure(s): food stimuli in the home (from Family Eating and Activity questionnaire), parent's weight | |||
| Other outcome measure(s): ‐ | Other outcome measure(s): ‐ | |||
| Aragona 1975 | Source: N/T | Primary outcome measure(s): ‐ | Primary outcome measure(s): ‐ | |
| Secondary outcome measure(s): ‐ | Secondary outcome measure(s): ‐ | |||
| Outcomes not stated as primary or secondary: weight, height | Other outcome measure(s): weight change | |||
| ‐ denotes not reported aTrial document(s) refers to all available information from published design papers and sources other than regular publications (e.g. FDA/EMA documents, manufacturer's website's, trial registers) bPublication(s) refers to trial information published in scientific journals (primary reference, duplicate publications, companion documents or multiple reports of a primary study) ACTRN: Australian New Zealand Clinical Trials Registry; BMA: body mass index; EMA: European Medicines Agency; FDA: Food and Drug Administration (US); ISRCTN: International Standard Randomised Controlled Trial Number; LDL: low‐density lipoprotein; N/A: not applicable; N/T: no trial document available; TV: television | ||||
Appendix 6. Examination of outcome reporting bias according to ORBIT classification
| Outcome | High risk of bias (category A)a | High risk of bias (category D)b | High risk of bias (category E)c | High risk of bias (category G)d | |
| Resnicow 2015 | N/A | ||||
| Mazzeo 2014 | N/A | ||||
| van Grieken 2013 | N/A | ||||
| Small 2013 | N/A | ||||
| Esfarjani 2013 | N/A | ||||
| Moens 2012 | N/A | ||||
| Raynor 2012a | N/A | ||||
| Raynor 2012b | N/A | ||||
| Magarey 2011 | N/A | ||||
| Jansen 2011 | N/A | ||||
| Collins 2011 | N/A | ||||
| Boutelle 2011 | N/A | ||||
| West 2010 | N/A | ||||
| Resnick 2009 | N/A | ||||
| Estabrooks 2009 | N/A | ||||
| Munsch 2008 | N/A | ||||
| Janicke 2008 | N/A | ||||
| Golley 2007 | N/A | ||||
| Golan 2006 | N/A | ||||
| Aragona 1975 | N/A | ||||
|
aClear that outcome was measured and analysed; trial report stated that outcome was analysed but only reports that result was not significant
(Classification 'A', table 2, Kirkham 2010)
bClear that outcome was measured and analysed; trial report stated that outcome was analysed but no results reported
( Classification 'D', table 2, Kirkham 2010)
cClear that outcome was measured; clear that outcome was measured but not necessarily analysed; judgement says likely to have been analysed but not reported because of non‐significant results
(Classification 'E', table 2, Kirkham 2010)
dUnclear whether the outcome was measured; not mentioned but clinical judgement says likely to have been measured and analysed but not reported on the basis of non‐significant results
(Classification 'G', table 2, Kirkham 2010) N/A: not applicable | |||||
Appendix 7. Definition of endpoint measurement
| Behaviour change | Changes in BMI and body weight | Height | Health‐related quality of life or self esteem | All‐cause mortality/morbidity | Socioeconomic effects | Parent‐child relationship or assessment of parenting | Participants' views of the intervention | Severe/serious adverse events | |
| Resnicow 2015 | Parental questionnaire for behavioural outcomes, not validated | Primary care physicians and assistants trained in assessment of height and weight and provided with print and online resources to convert heights and weights to BMI and BMI percentile. All practices were provided with a digital scale. Parent BMI was calculated from self reported heights and weights | All practices were provided with a 36‐inch calibration rod and, if needed, a new stadiometer | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Mazzeo 2014 | Parents completed the questionnaire ‐ dietary intake was assessed by using the Block Food Screener. This instrument has been validated against the Block 100‐item FFQ. CFQ: parental approaches to and attitudes about feeding their children were measured with the CFQ. This measure includes 7 subscales: perceived responsibility, perceived parent weight, perceived child weight, concern about child weight, pressure to eat, monitoring, and restriction | Height was measured to the nearest quarter of an inch. using a stadiometer. Weight was measured to the nearest quarter of a pound; data were used to calculate BMI in kg/m2, which were plotted on the CDC growth charts to obtain BMI percentile for age and gender | ‐ | See Appendix 11 | ‐ | ‐ | Parental concern subscale of the CFQ 3 questions, 5‐point scale (1‐5) , higher scores equate to more concern |
Participants will complete an exit questionnaire that assesses: what they liked and disliked about the intervention; thoughts about the duration, frequency, and number of sessions; perceived benefits and barriers to implementing the intervention goals; comfort with group leaders and members; overall satisfaction; and suggestions for improvement | ‐ |
| van Grieken 2013 | Parent report of child health behaviour (unvalidated, not extracted) | Weight was assessed via questionnaire that was completed by the parent. Also the YHC professionals measured at baseline. At follow‐up either the YHC professionals or a research assistant measured using the same standardized methods and equipment. Weight measured to the nearest 0.1 kilogram. BMI calculated by weight/height and children were classified into normal, overweight or obesity according to international age and gender specific cut‐off points (reference provided). Child BMI Standard Deviation Scores (SDS) were calculated using the reference population of children from the 1997 Dutch national Growth study |
Height was assessed via questionnaire which was completed by the parent. Also the YHC professional measured at baseline. At follow‐up either the YHC professionals or a research assistant measured using the same standardised methods and equipment. Height measured to the nearest 0.1 cm. Waist circumference measured over naked skin at the level midway between the lower rib margin and the iliac crest at the end of gentle expiration when the child was standing |
‐ | ‐ | ‐ | Parenting practices were assessed by unvalidated measures (not data extracted) | Questionnaire assessing acceptability and feasibility of the protocol were sent after the first or second additional session. Parents were asked to indicate if the information provided was appreciated | ‐ |
| Small 2013 | ‐ | Weight was assessed in pounds (scale displayed to the
nearest one hundredth decimal place) using a portable Tannita scale. BMI percentile was derived from the BMI z score using the normal distribution function in Microsoft Excel 2003 |
Height assessed to the nearest eighth inch using a Seca portable stadiometer | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Esfarjani 2013 | The validated food frequency questionnaire was used to assess typical food intake over the previous year (not data extracted) Unvalidated measures of TV watching, computer use (not data extracted) |
Weight was measured by trained experts to the nearest 0.1 kg using a calibrated and certified portable digital scale with lightly dressed, without shoes and empty pockets. BMI was calculated (kg/m2 ) Waist circumference was measured at the smallest area between the edge of the lower chest and iliac crest bone |
Height was measured by trained experts and determined in a standing position, barefoot using a portable height gauge with accuracy of 0.1 cm |
‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Moens 2012 | Dutch Eating Behaviour Questionnaire (DEBQ, child and parent versions) ‐ validated measure with 33 items scored on a 5‐point Likert scale. Child version phrasing adapted slightly. Health principles questionnaire ‐ developed for the study, not validated (not data extracted) |
Weight in light clothing by a school physician on a balance‐beam scale. Post intervention and follow‐up measured by parental report. Adjusted BMI (actual BMI/percentile 50 of BMI for age and gender x 100) calculated and overweight or obese identified in relation to European norms for 0‐21 year olds. Also BMI percentiles and z scores calculated by US CDC |
Height without shoes measured by a wall‐mounted stadiometer. Post intervention and follow‐up measured by parental report | ‐ | ‐ | ‐ | Ghent Parental Behaviour Scale (GPBS), validated tool, 9 scales | ‐ | ‐ |
| Raynor 2012a | Parents were asked to complete diaries for their children 3 days each week (1 weekend day, 2 weekdays). Leisure‐time activity assessed via The Previous Day Physical Activity Recall (PD‐PAR). Parents were asked to complete the PD‐PAR for their children | By trained research staff blinded to treatment assignment. Weight was assessed by a balance beam scale, and height was assessed using a stadiometer, using standard procedures (16) with participants wearing light clothing and no shoes. BMI was calculated with the following formula: BMI = weight in kg/height in m2. For children, standardised BMI (BMI‐z) scores were calculated based upon the value of the 50th BMI percentile and the standard deviation of the age‐ and sex‐ appropriate sample from the CDC growth charts | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Raynor 2012b | ‐ | By trained research staff blinded to treatment assignment. Weight was assessed by a balance beam scale, and height was assessed using a stadiometer, using standard procedures (16) with participants wearing light clothing and no shoes. BMI was calculated with the following formula: BMI = weight in kg/height in m2. For children, standardised BMI (BMI‐z) scores were calculated based upon the value of the 50th BMI percentile and the standard deviation of the age‐ and sex‐ appropriate sample from the CDC growth charts | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Magarey 2011 | ‐ | BMI measured using standard anthropometric methods, and BMI z scores were calculated using 1990 UK reference data child weight status as determined using IOTF definition | ‐ | ‐ | ‐ | ‐ | Alabama Parenting Questionnaire (Satisfaction, Efficacy, Involvement, Positive parenting, Poor monitoring, Inconsistent discipline, Corporal punishment) 35 questions, higher scores indicate improvement |
‐ | ‐ |
| Jansen 2011 | A self report questionnaire. Child Eating Disorder Examination Questionnaire, which is based on the Eating Disorder Examination Questionnaire. Eating behaviour by interview, Scores for regularity range from 0 to 7, a higher score indicating a more regular eating pattern. Physical activity: filled out by overweight child (in presence of a parent and the interviewer). This questionnaire consists of 3 components: work activity, sports activity and leisure activity. An index score for the 3 components is calculated, ranging from 1 to 5, with higher index scores reflecting higher levels of activity. The Baecke Questionnaire was adapted for use with children by replacing 'work activity' with 'school activity' (including similar questions) | Weight (kg) measured and BMI percentile (Children's BMI‐for‐Age Calculator) was calculated for each child of the participating parents, thus including the target children and their siblings | ‐ | Self esteem: overweight child filled out the questionnaire (in presence of a parent and the interviewer). Perceived competence was measured with the translated version of the Self‐Perception Profile for Children. Negative thoughts: completed by the overweight child (in presence of a parent and the interviewer). This questionnaire maps the frequency of negative weight‐related thoughts in overweight children. The questionnaire comprises 20 items (for example 'I am worthless because I am overweight'). Scores range from 1 ('I never have this thought') to 5 ('I always have this thought') | ‐ | ‐ | ‐ | ‐ | ‐ |
| Collins 2011 | Habitual physical activity was measured using the Actigraph 7164 uniaxial accelerometer, an objective measure of activity. Participants wore the accelerometer during all waking hours over 8 consecutive days. Parents and children recorded periods
of non‐wear. The screen behaviours sub‐scale of the Children's Leisure Activities Study Survey completed by parents to assess children's time spent in TV/DVD viewing, playing electronic games, and using the computer |
Weight ass measured with the children barefoot and wearing light clothing, using Tanita HD646 scales (Tanita Corporation of America Inc, Illinois, USA) to 0.1 kg | Height was measured to 0.1 cm using the stretch stature method and PE87 portable stadiometers (Mentone Educational Centre, Victoria, Australia) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Boutelle 2011 | Usual dietary intake of the child was assessed with the Block Kids Questionnaire. Children completed the Physical Activity Questionnaire for Older Children (PAQ‐C) | Weight measured in kg on a Tanita Digital Scale. BMI was standardised for age and gender (BMI z score) and expressed as a percentile (BMI percentile) using the US CCDC Growth Curves |
Height was measured using a portable Schorr height board | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| West 2010 | The Lifestyle Behaviour Checklist lists 25 child problem behaviours related to eating (e.g. eating too quickly), physical activity (e.g. playing too many computer games) and overweight (e.g. complaining about being teased) in children aged 4‐11 years and yields scores on 2 scales ‐ the Problem scale and Confidence scale. For the Problem scale, parents are asked to rate the extent to which they experience each of these behaviours as a problem with their child (higher scores indicate greater problems). For the Confidence scale, parents are asked to rate how confident they feel managing each of these behaviours, even if not currently occurring (higher scores indicate greater confidence). The recommended clinical cut‐offs are a Problem scale score > 50 (range 25‐175) and a confidence scale score < 204 (range 25‐250). The Parenting Scale: for each of the 30 items, parents are asked to rate how they would respond to a given discipline situation by choosing between an effective or ineffective course of action. The ineffective parenting practices assessed by the Problem scale include permissive or inconsistent discipline (e.g. coaxing or begging their child to stop a problem behaviour), coercive discipline (e.g. spanking their child), and emotional discipline and irritability (e.g. getting angry or upset when their child misbehaves) | Height and weight were measured with a custom‐made portable stadiometer and electronic scales using standard procedures. BMI z scores were calculated using parameters published by the CDC | ‐ | ‐ | ‐ | ‐ | ‐ | Client Satisfaction Questionnaire | ‐ |
| Resnick 2009 | Parents were asked whether they received information about their children's nutrition or physical activity from any of the presented sources. Parents were asked to state their confidence, Response categories were 'not at all confident', 'a little confident', 'somewhat confident', 'confident', or 'very confident'. These responses were collapsed into dichotomous categories of confident and very confident vs. not at all confident, a little confident, and somewhat confident. Parents were asked to report the mean number of hours per day that both they and their children watched TV or videos during the 30 days prior to completing the baseline and post‐intervention surveys. Parents were also asked about the number of servings of fruits and vegetables that both they and their children consumed during the 30 days prior to completing the baseline and post‐intervention surveys. Both TV and nutritional responses were collapsed into dichotomous categories of those who consumed ≥ 5 servings of fruit and vegetables per day vs. those who consumed < 5 servings | BMI collected standardised by school taken by school nurse. BMI was calculated and re‐scaled to represent the percentage of BMI measures for that age group |
‐ | ‐ | ‐ | ‐ | ‐ | Parent satisfaction: parents were asked whether they read the study materials, whether they found at least 1 material to be helpful, and whether they would recommend the programme to other families. Parents in the educational material plus personal encounters (intervention 2) group were also asked whether they found their community health workers to be helpful | ‐ |
| Estabrooks 2009 | ‐ | Weight was assessed using a regularly calibrated medical scale | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Munsch 2008 | Description of validated measures consistent with psychology/psychometrics. The Child Behavior Checklist for children and adolescents aged 4‐18 years recorded the assessments of parents in terms of social skills and problems of emotional well‐being and behaviour of their children the total value of internalising and externalising subscales, as well as the syndrome scale "Social problems" were recorded, representing a relevant concept in obese children |
BMI measures taken and consistent with others, e.g. weight on a secca e scale. Percent overweight = [(effective BMI/BMI 50th percentile) ‐ 1] was calculated based on age and gender | Stadiometer to measure height | All children completed questionnaires to assess depressive symptoms (Children's Depression Inventory; DIKJ), anxiety (STAIK; SASC‐R and its German version), SAD, and FNE | ‐ | ‐ | ‐ | ‐ | ‐ |
| Janicke 2008 | Parents were asked to help their children complete the measure. The Youth/Adolescent Food Frequency Questionnaire was used to assess the child's dietary intake during the preceding month | Height without shoes measured to the nearest 0.1 cm using a Harpendon stadiometer. Weight to nearest 0.1 kg with 1 layer of clothing on and without shoes using a calibrated balance bean scale. Measured 3 times and averaged | Measured without shoes to nearest 0.1 cm using a Harpendon stadiometer (Holtain Ltd, Crosswell, UK). Measured 3 times and averaged | Children completed, Self‐Perception Profile for Children, which is a self report assessment of the child's perception of his or her global self worth and competence in 6 specific domains: scholastic competence, social acceptance, athletic competence, physical appearance, behavioural conduct, and global self worth | ‐ | ‐ | ‐ | ‐ | ‐ |
| Golley 2007 | ‐ | Weight was measured to the nearest 0.1 kg with "SECA" electronic scales. BMI z: BMI was calculated and converted to a BMI z score by using UK reference data provided as a computer program (Child Growth Foundation, London, UK) |
‐ | ‐ | ‐ | ‐ | ‐ | Validated, anonymous 16‐item questionnaire adapted from the one usually used as part of the Triple P programme | ‐ |
| Golan 2006 | ‐ | BMI: weight and height were measured to the nearest 0.1 kg and 1 cm, respectively, using a standard medical balance‐beam scale with a rigid vertical height rod | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Aragona 1975 | ‐ | Used bathroom scales. 3 baseline measurements obtained over a 2‐week period for both experimental groups. Control group were measured at home on the same day. To ensure reliability the experimenters independently read all height and weight measurements in the presence of the parents. If there were any inter‐rater discrepancies on weight, the child was weighed again. Weights recorded when there was inter‐rater agreement on 2 consecutive measurements | Not reported how measured | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| "‐" denotes not reported. 1 inch (in) = 2.5 cm; 1 pound (lb) = 450 g BMI: body mass index; CDC: Centers for Disease Control and Prevention; CFQ: Child Feeding Questionnaire; cm: centimetre; DIKJ: Depressionsinventar für Kinder und Jugendliche; FFQ: Food Frequency Questionnaire; FNE: Fear of Negative Evaluation; IOTF: International Obesity Task Force; kg: kilogram; lb: pound; PAQ‐C: Physical Activity Questionnaire for Older Children; PD‐PAR SDS: Previous Day Physical Activity Recall; SAD: Social Avoidance and Distress; SASC‐R: Social Anxiety Scale for Children‐Revise; STAIK: State und Trait Angst‐Inventar für Kinder; TV: television; YHC: Youth Health Centre | |||||||||
Appendix 8. Adverse events
| Intervention(s) and comparator(s) | Participants included in analysis [N] | Deaths [N (%)] | Participants with adverse events [N (%)] | Participants with severe/serious adverse events [N (%)] | Participants discontinuing study due to adverse events [N / %] | Participants hospitalised [N (%)] | Participants with outpatient treatment [N (%)] | Participants with specific adverse events [description] [N /%] | |
| Resnicow 2015 | I1: parent‐only PCP motivational interviewing | 16 practices 212 participants |
‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| I2: parent‐only PCP + dietician motivational interviewing | 15 practices 235 participants |
‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| C: usual care | 11 practices 198 participants |
‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Mazzeo 2014 | I: parent NOURISH | 48 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: parent control | 45 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| van Grieken 2013 | I: parent‐only | 349 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: usual care | 288 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Small 2013 | I: parent‐only | 34 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: control | 33 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Esfarjani 2013 | I: parent‐only | 70 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: control | 86 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Moens 2012 | I: parent‐only | 27 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: control | 19 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Raynor 2012a | I: parent‐only | 33 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C1: parent ‐ diet decrease | 33 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| C2: parent ‐ diet increase | 35 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Raynor 2012b | I: parent‐only | 29 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C1: parent ‐ diet and activity traditional | 26 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| C2: parent ‐ diet and activity substitute | 26 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Magarey 2011 | I: parent healthy lifestyle | 85 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: healthy lifestyle | 84 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Jansen 2011 | I: parent CBT | 59 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: waiting list control | 39 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Collins 2011 | I: parent‐only diet | 63 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C1: parent‐child (physical activity) | 73 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| C2: parent‐child (physical activity + diet) | 70 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Boutelle 2011 | I: parent‐only | 40 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: parent‐child | 40 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| West 2010 | I: parent‐only | 52 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: control | 49 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Resnick 2009 | I: educational material + personal encounters | 22 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: educational material | 24 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Estabrooks 2009 | I: parent group + IVR | 85 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| I2: parent group | 85 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| C: parent workbook | 50 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Munsch 2008 | I: mother only | 25 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: mother‐child | 31 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Janicke 2008 | I: parent‐only | 34 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C1: parent‐child | 33 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| C2: waiting list control | 26 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Golley 2007 | I: parent + lifestyle education | 38 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C1: parent | 37 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| C2: waiting list control | 36 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Golan 2006 | I: parent‐only | 14 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: parent‐child | 18 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Aragona 1975 | I1: parent‐only + reinforcement | 5 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| I2: parent‐only | 5 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| C: control | 5 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| "‐" denotes not reported C: comparator; CBT: cognitive behavioural therapy; I: intervention; IVR: interactive voice response; NOURISH: Nourishing Our Understanding of Role Modelling to Improve Support and Health; PCP: primary care providers | |||||||||
Appendix 9. Checklist to aid consistency and reproducibility of GRADE assessments
| Body mass index (BMI) variables | ||
| Study limitations (risk of bias)a | 1. Was random sequence generation used (i.e. no potential for selection bias)? | Yes |
| 2. Was allocation concealment used (i.e. no potential for selection bias)? | Unclear | |
| 3. Was there blinding of participants and personnel (i.e. no potential for performance bias)? | Unclear | |
| 4. Was there blinding of outcome assessment (i.e. no potential for detection bias)? | Unclear | |
| 5. Was an objective outcome used? | Yes | |
| 6. Were > 80% of participants enrolled in trials included in the analysis (i.e. no potential reporting bias)? | No (↓) | |
| 7. Were data reported consistently for the outcome of interest (i.e. no potential selective reporting)? | No (↓) | |
| 8. No other biases reported (i.e. no potential of other bias)? | Yes | |
| 9. Did the trials end up as scheduled (i.e. not stopped early)? | Yes | |
| Inconsistencyb | 1. Point estimates did not vary widely? | Yes |
| 2. To what extent did confidence intervals overlap (substantial: all confidence intervals overlap at least 1 of the included studies point estimate; some: confidence intervals overlap but not all overlap at least 1 point estimate; no: at least 1 outlier: where the confidence interval of some of the studies do not overlap with those of most included studies)? | Substantial | |
| 3. Was the direction of effect consistent? | No | |
| 4. What was the magnitude of statistical heterogeneity (as measured by I2) ‐ low (I2 < 40%), moderate (I2 40‐60%), high I2> 60%)? | Low | |
| 5. Was the test for heterogeneity statistically significant (P < 0.1)? | Not statistically significant | |
| Indirectnessc | 1. Were the populations in included studies applicable to the decision context? | Highly applicable |
| 2. Were the interventions in the included studies applicable to the decision context? | Highly applicable | |
| 3. Was the included outcome not a surrogate outcome? | No (however, only relevant outcome) | |
| 4. Was the outcome timeframe sufficient? | Sufficient | |
| 5. Were the conclusions based on direct comparisons? | Yes | |
| Imprecisiond | 1. Was the confidence interval for the pooled estimate consistent with benefit? | No (↓) |
| 2. What is the magnitude of the median sample size (high: > 300 participants, intermediate: 100‐300 participants, low: < 100 participants)?e | Low to intermediate (↓) | |
| 3. What was the magnitude of the number of included studies (large: > 10 studies, moderate: 5‐10 studies, small: < 5 studies)?e | Small (↓) | |
| 4. Was the outcome a common event (e.g. occurs more than 1/100)? | N/A | |
| Publication biased | 1. Was a comprehensive search conducted? | Yes |
| 2. Was grey literature searched? | Yes | |
| 3. Were no restrictions applied to study selection on the basis of language? | Yes | |
| 4. There was no industry influence on studies included in the review? | Yes | |
| 5. There was no evidence of funnel plot asymmetry? | Unclear | |
| 6. There was no discrepancy in findings between published and unpublished trials? | Unclear | |
|
aQuestions on risk of bias are answered in relation to the majority of the aggregated evidence in the meta‐analysis rather than to individual trials.
bQuestions on inconsistency are primarily based on visual assessment of forest plots and the statistical quantification of heterogeneity based on I2. cWhen judging the width of the confidence interval it is recommended to use a clinical decision threshold to assess whether the imprecision is clinically meaningful. dQuestions address comprehensiveness of the search strategy, industry influence, funnel plot asymmetry and discrepancies between published and unpublished trials. eDepends on the context of the systematic review area. (↓): key item for possible downgrading the quality of the evidence (GRADE) as shown in the footnotes of the 'Summary of finding' table(s); GRADE: Grading of Recommendations Assessment, Development and Evaluation; N/A: not applicable. | ||
Appendix 10. Survey of study investigators providing information on included trials
| Study author contacted (DD/MM/YYYY) | Study author replied (DD/MM/YYYY) | Study author asked for additional information (short summary) | Study author provided data (short summary) | |
| Resnicow 2015 | 15/11/2015 | ‐ | ‐ | ‐ |
| Mazzeo 2014 | 10/08/2015 | 10/08/2015 | Allocation concealment approach and blinding of outcome assessors | ‐ |
| van Grieken 2013 | 10/08/2015 | 10/08/2015 | Allocation concealment approach and selective reporting of outcomes | Yes, 14/09/2015 |
| Small 2013 | 11/08/2015 | ‐ | N/A | N/A |
| Esfarjani 2013 | 11/08/2015 | ‐ | N/A | N/A |
| Moens 2012 | 18/08/2015 | 25/08/2015 | Details blinding of participants, personnel and outcome assessors | Yes, 03/09/15 |
| Raynor 2012a | 20/11/2014 | 25/11/2014 | Mean and standard deviation of the BMI z scores for each of the 3 arms in the trial | Yes, 25/11/2014 |
| Raynor 2012b | 20/11/2014 | 25/11/2014 | Mean and standard deviation of the BMI z scores for each of the3 arms in the trial | Yes, 25/11/2014 |
| Magarey 2011 | 11/08/2015 | 11/08/2015 | N/A | N/A |
| Jansen 2011 | 11/08/2015 | 11/08/2015 | Randomisation procedures, blinding of outcome assessors, selective reporting of outcomes | ‐ |
| Collins 2011 | 11/08/2015 | 11/08/2015 | Allocation concealment approach | ‐ |
| Boutelle 2011 | 11/08/2015 | ‐ | N/A | N/A |
| West 2010 | 11/08/2015 | 12/08/2015 | Selective reporting of outcomes | ‐ |
| Resnick 2009 | 11/08/2015 | ‐ | N/A | N/A |
| Estabrooks 2009 | 11/08/2015 | ‐ | N/A | N/A |
| Munsch 2008 | 16/04/2014 | 16/04/2014 | Additional data for Child Behaviour Checklist and English version of study publication | Yes, 16/04/2014 |
| Janicke 2008 | 11/08/2015 | 11/08/2015 | Allocation concealment approach, blinding of outcome assessors | Yes, 24/08/15 |
| Golley 2007 | 11/08/2015 | 11/08/2015 | N/A | N/A |
| Golan 2006 | 11/08/2015 | ‐ | N/A | N/A |
| Aragona 1975 | No email address | ‐ | N/A | N/A |
| N/A: not applicable | ||||
Appendix 11. Health‐related quality of life: instruments
| Name (type of measurement) | Dimensions (subscales) (number of items) | Validated instrument | Answer options | Scores | Direction of scales | Minimal important difference | |
| Mazzeo 2014 | Pediatric Health‐Related Quality of Life (PedsQL4.0) | 4 (physical (8 items), emotional (5 items), social (5 items), and school (5 items)) | Yes | 5‐point Likert scale from 0 (never) to 4 (almost always) | Scores are transformed on a scale from 0 to 100 | Higher scores indicate better HRQoL | Unknown |
| HRQoL: health‐related quality of life | |||||||
Data and analyses
Comparison 1. Parent‐only interventions versus parent‐child interventions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 BMI z score change post intervention | 3 | 277 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.13, 0.02] |
| 1.1 Parent‐only vs. parent‐child | 2 | 112 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.13, 0.04] |
| 1.2 Parent‐only vs. parent‐child physical activity | 1 | 84 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.26, ‐0.04] |
| 1.3 Parent‐only vs. parent‐child physical activity + diet | 1 | 81 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.11, 0.11] |
| 2 BMI z score change longest follow‐up | 3 | 267 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.15, 0.08] |
| 2.1 Parent‐only vs. parent‐child | 2 | 102 | Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.05, 0.16] |
| 2.2 Parent‐only vs. parent‐child physical activity | 1 | 84 | Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.36, 0.04] |
| 2.3 Parent‐only vs. parent‐child physical activity + diet | 1 | 81 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.31, 0.09] |
Comparison 2. Parent‐only interventions versus waiting list interventions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 BMI z score change post intervention | 2 | 153 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.21, ‐0.04] |
| 2 BMI z score change longest follow‐up | 2 | 136 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.19, ‐0.01] |
| 2.1 Parent‐only vs. waiting list | 2 | 92 | Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.21, ‐0.01] |
| 2.2 Parent‐only intensive education vs. waiting list | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.29, 0.25] |
| 3 BMI percentile change post intervention | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 BMI percentile change longest follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 BMI change post intervention | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Parent‐only reinforcement vs. waiting list | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Parent‐only vs. waiting list | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 BMI change longest follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Parent‐only reinforcement vs. waiting list | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Parent‐only vs. waiting list | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. Parent‐only interventions versus minimal contact interventions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 BMI z score change post intervention | 1 | 170 | Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.08, 0.08] |
| 1.1 Parent‐only IVR vs. control | 1 | 87 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.13, 0.09] |
| 1.2 Parent‐only vs. control | 1 | 83 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.09, 0.13] |
| 2 BMI z score change longest follow‐up | 1 | 165 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.07, 0.09] |
| 2.1 Parent‐only interactive voice response vs. control | 1 | 86 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.13, 0.09] |
| 2.2 Parent‐only vs. control | 1 | 79 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.07, 0.15] |
| 3 BMI percentile change post intervention | 4 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Parent‐only vs. minimal contact control | 3 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Parent motivational interviewing vs. minimal contact control | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Parent motivational interviewing + dietician vs. minimal contact control | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 BMI percentile change longest follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 BMI change post intervention | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6 BMI change longest follow‐up | 2 | 614 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.39, 0.15] |
3.1. Analysis.
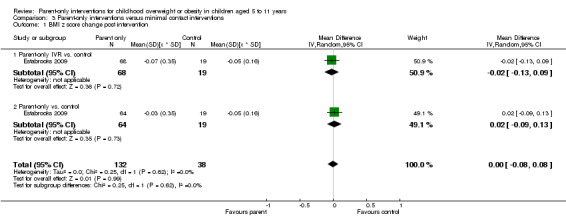
Comparison 3 Parent‐only interventions versus minimal contact interventions, Outcome 1 BMI z score change post intervention.
Comparison 4. Parent‐only intervention versus parent‐only intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 BMI z score change post intervention | 5 | 507 | Mean Difference (IV, Fixed, 95% CI) | ‐0.22 [‐0.28, ‐0.17] |
| 1.1 Parent‐only interactive voice response vs. parent‐only | 1 | 132 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.16, 0.08] |
| 1.2 Parent‐only intensive vs. parent‐only | 1 | 57 | Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐0.38, 0.20] |
| 1.3 Parent health lifestyle vs. healthy lifestyle | 1 | 136 | Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐0.29, 0.15] |
| 1.4 Parent‐only vs. decrease | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.17, 0.09] |
| 1.5 Parent‐only vs. increase | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.14, 0.12] |
| 1.6 Parent‐only vs. substitute | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐0.70 [‐0.86, ‐0.54] |
| 1.7 Parent‐only vs. traditional | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | ‐0.69 [‐0.83, ‐0.55] |
| 2 BMI z score change longest follow‐up | 5 | 467 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.10, 0.03] |
| 2.1 Parent‐only interactive voice response vs. parent‐only | 1 | 119 | Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.18, 0.06] |
| 2.2 Parent‐only intensive vs. parent‐only | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐0.32, 0.14] |
| 2.3 Parent health lifestyle vs. healthy lifestyle | 1 | 106 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.24, 0.30] |
| 2.4 Parent‐only vs. decrease | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.19, 0.11] |
| 2.5 Parent‐only vs. increase | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.17, 0.13] |
| 2.6 Parent‐only vs. substitute | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.24, 0.18] |
| 2.7 Parent‐only vs. traditional | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.17, 0.19] |
| 3 BMI change post intervention | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 BMI change longest follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 BMI percentile change post intervention [%] | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [author‐defined order]
Resnicow 2015.
| Methods |
Cluster randomised controlled trial Randomisation ratio: initially 1 : 1 : 1, final 5 sites randomised 1 : 2 : 2 owing to higher drop‐out in the practices providing the 2 interventions Superiority design |
|
| Participants |
Inclusion criteria: aged 2‐8 years with a BMI ≥ 85th and ≤ 97th percentile based on Centers for Disease Control cut‐points (reference provided) Exclusion criteria: type 1 or type 2 diabetes, non‐English‐speaking parent, no working telephone, chronic medical disorders, chromosomal disorders, syndromes and non‐ambulatory conditions, medications known to affect growth, enrolment in a weight loss programme, seen by weight loss specialist in past 12 months Diagnostic criteria: as above |
|
| Interventions |
Number of study centres: ‐ Treatment before study: ‐ Titration period: ‐ Description of interventions: BMI2 (Brief Motivational Interviewing to reduce body mass index): 1. Moderate‐intensity (4 sessions, 3 in year 1) primary care providers (PCP) motivational interviewing (MI) based counselling. PCPs received 2 days of in‐person training in MI and behaviour therapy and an interactive MI DVD training. Provided counselling sessions with a parent of the index child in year 1 and 1 additional "booster" visit in year 2 as well as usual care (described below). MI uses specific techniques such as reflective listening, autonomy support, shared decision‐making, and eliciting change talk. Focused on discrete behaviours, such as snack foods, sweetened beverages, fruits, vegetables, TV/screen time and physical activity/exercise. Provided positive feedback for healthy behaviours and then, collaboratively with the parent, identify behaviours that might be modified. Pre‐existing or new materials written in a style consistent with MI and self determination theory. Content emphasised child choice in making behaviour change. Self monitoring logs could be used, clinicians educational materials and logs specific to the family 2. High‐Intensity, PCP and dietician, intervention. Same intervention components as moderate‐intensity group (4 sessions with PCP) but added 6 MI‐based counselling (4 in year 1) from a trained dietician. Sessions were delivered either in‐person or by telephone. Dieticians given same training in MI as the PCPs 3. Usual care: routine care by the PCP, as well as standard educational materials for parents on healthy eating and exercise. Practitioners attended a half‐day orientation session that included current treatment guidelines PCPs and dieticians were incentivised for the number of sessions and the number of participants recruited additional payments for retaining 50% and 80% of cohort |
|
| Outcomes | Outcomes reported in abstract of publication: BMI percentile | |
| Study details |
Run‐in period: ‐ Study terminated before regular end (for benefit/because of adverse events): no |
|
| Publication details |
Language of publication: English Non‐commercial funding Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "to test the efficacy of moderate‐intensity (4 sessions) PCP MI‐based counselling and the effect of adding 6 MI‐based counselling sessions by trained dietitians delivered to parents of overweight youth aged 2 to 8 years recruited through primary care offices" | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from publication: "randomly assigned" Comment: no details |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Quote from publication: "open label" Comment: investigator‐assessed |
| Blinding of outcome assessment (detection bias) Objective outcome | Low risk | Quote from publication: "open label" Comment: investigator‐assessed, low risk of bias from objective outcomes |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | Comment: reported with reasons per practice |
| Selective reporting (reporting bias) | High risk | Comment: no secondary outcomes reported |
| Other bias | Low risk | Comment: no other bias |
Mazzeo 2014.
| Methods |
Parallel randomised controlled trial Randomisation ratio: not reported Superiority design |
|
| Participants |
Inclusion criteria: BMI ≥ the 85th percentile who primarily resided with the participating carer. Participants also had to speak English, be able to understand basic instructions and perform simple exercises Exclusion criteria: carers: non‐ambulatory, pregnant or have a medical diagnosis that may be negatively impacted by exercise. Parents whose children have a medical condition or developmental disorder that precludes weight loss using conventional diet and exercise methods Diagnostic criteria: BMI ≥ 85th percentile |
|
| Interventions |
Number of study centres: not reported Treatment before study: none Titration period: none Description of interventions NOURISH parenting intervention Based in Social Cognitive Theory (SCT), and emphasises parental role modelling as a primary way children learn health behaviours. Focuses on enhancing parents' self efficacy to make positive changes in eating and exercise behaviours. In addition, cognitive‐behavioural strategies such as self monitoring, contingency management and stimulus control incorporated. All sessions involved participatory activities, including self assessments, group discussions and experiential activities. These participatory experiences aimed to enhance overall intervention efficacy The intervention also informed by Stages of Change Theory and is integrated into the intervention through regular self assessment of barriers to change as well as moderated discussions on the process (pros and cons) of engaging in a healthier lifestyle. Focus on the parents' relationship with everyone in the family, not just the "identified patient" or overweight child, as recommended by Golan and colleagues Outline of NOURISH session content (each session was 90 minutes): Session 1 ‐ overview of childhood eating problems and becoming an empowered parent; session 2 ‐ the "toxic environment": how can parents fight back?; Session 3 ‐ nutrition, portion sizes, fruits and vegetables; session 4 ‐ emotional and mindful eating; session 5 ‐ parenting styles; session 6 ‐ helping your child develop a healthy relationship with food; session 7 ‐ increasing physical activity; session 8 ‐ reducing physical activity barriers; session 9 ‐ promoting a healthy body image; session 10 ‐ dealing with teasing; session 11 ‐ raising a media‐savvy child; session 12 ‐ bringing it all together All sessions led by doctoral students in psychology working under the supervision of a licensed, clinical psychologist with specific training in group facilitation. Sessions were video‐recorded to allow the investigators to monitor treatment fidelity. Interventionists met weekly with the principal investigator for supervision. These meetings were used to review interventionists' adherence to the treatment protocol, to review programme retention, and to discuss participant interactions. Parents had a 1‐hour booster session 2 months after the intervention to allow parents to share with one another their successes, and to elicit suggestions from group leaders and fellow parents regarding barriers they have encountered Control parenting intervention Parents in the control group attended a group session moderated by an independent interventionist (a doctoral student in psychology). The session addressed the role of diet and exercise in paediatric overweight. Control participants were mailed publicly available brochures on paediatric overweight on 3 occasions during the study: between weeks 4 and 5, between weeks 8 and 9, and 2 months after post‐testing (the latter of which was meant to match the NOURISH booster session) Parents and children in both the intervention and control groups received a pedometer. Intervention parents also received a raffle ticket at each session for a USD 75 gift card, which will take place at the final session. Participants who attend the final session were given Certificates of Completion. All parents (i.e. intervention and control groups) were given USD 20 gift cards for completing the pre‐test, post‐test and the 6‐month follow‐up. The study provided childcare for all programme sessions and assessments |
|
| Outcomes | Outcomes reported in abstract of publication: child BMI, parents satisfaction, parent behaviour change | |
| Study details |
Run‐in period: none Study terminated before regular end (for benefit/because of adverse events): no |
|
| Publication details |
Language of publication: English Non‐commercial funding Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "The primary aim of this study is to implement and evaluate the feasibility, acceptability, and preliminary effectiveness of NOURISH, a culturally sensitive, parent‐only skills‐based group intervention, and a single‐session, education‐only, control group (parent‐only) intervention on overweight children's BMI percentile. The secondary aim of this study is to evaluate the effectiveness of the intervention for improving children's dietary intake, body dissatisfaction, and quality of life. The impact of these two programs on adult participants will also be evaluated, including parental BMI and dietary intake. Parent satisfaction and feedback regarding the NOURISH intervention will also be elicited..." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: "Participants will be randomly assigned (using a random number generator) to one of two parent‐only interventions..." |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details provided |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Unclear risk | Comment: no details provided |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Comment: carers in the control arm were blinded to the aims and hypotheses of this study otherwise no details masking of treatment assignment |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Comment: no details provided |
| Blinding of outcome assessment (detection bias) Objective outcome | Unclear risk | Comment: no details provided |
| Incomplete outcome data (attrition bias) Subjective outcomes | Low risk | Comment: no details provided |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | Comment: numbers of drop‐outs and withdraws provided and similar across groups. ITT analysis ‐ analysed all the data according to participants' assigned group, whether or not they actually completed the intervention. Used participants' most recent data as post intervention scores |
| Selective reporting (reporting bias) | High risk | Comment: many outcomes were collected but not reported ‐ though it was reported that these were not significantly different from baseline |
| Other bias | Unclear risk | Comment: although it was made clear why the trial team changed the protocol intervention time from 12 weeks to 6 weeks, this did not seem to help retain participants and meant if there was to be an effect it could not be shown in the health‐related quality of life of participants' children |
van Grieken 2013.
| Methods |
Cluster randomised controlled trial Randomisation ratio: 1 : 1 Superiority design: |
|
| Participants |
Inclusion criteria: child classified as being overweight (not obese) according to international age and gender specific cut‐off points for BMI at the well‐child visit (country wide health visit in the year a child turns 5 years), attended between September 2007 and October 2008. Parents with at least basic Dutch language skills Exclusion criteria: obese children with chronic health problems that many influence outcomes Diagnostic criteria: not reported |
|
| Interventions |
Number of study centres: 9 (44 teams) Treatment before study: none Titration period: none Intervention description: Parent‐only intervention: motivational interviewing if needed, with information about overweight prevention and healthy lifestyle choices. Initiated at the well‐child visit and up to 3 structured counselling sessions could be offered at approximately 3, 6 and 12 months later. The session content depended on the stage of behavioural change of the parents (individually tailored). Motivation was assessed by Youth Health Care professionals by creating awareness of the child's weight status and associated consequences. 4 lifestyle‐related behaviours could be promoted: playing outdoors, eating breakfast, reducing sweet drinks and sedentary behaviour. Parents choose 1 or 2 behaviours to target during the sessions. Advice was by international guidelines. Information materials provided, diet and activity diaries discussed and family‐oriented action plans to change health‐related behaviour documented Youth Health care professionals were provided with a half‐day training in motivational interviewing techniques, were provided with a workbook with information and practical examples and an information sheet with step‐by‐step guide to how the information could be applied Control group: parents informed of overweight of their child but usual care (general information about a healthy lifestyle) given |
|
| Outcomes | Outcomes reported in abstract of publication: BMI, minutes of outside play or TV viewing, having breakfast, number drinks of sweet beverages | |
| Study details |
Run‐in period: none Study terminated before regular end (for benefit/because of adverse events): no |
|
| Publication details |
Language of publication: English Non‐commercial funding Publication status: peer‐review publication |
|
| Stated aim for study | Quote from publication: "Children who are overweight (not obese) visiting YHC teams…would have a less increase in BMI and waist circumference at follow‐up compared to overweight children visiting YHC teams allocated to the control condition, performing usual care" | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: "within each centre, YHC teams were randomised... by means of a computer‐generated random number list… random permuted blocks… lengths were 4 or 6 depending on the number of teams per Municipal Health Service" |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details of allocation concealment provided by study authors |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Quote from publication: "parents were not aware of the research condition they were allocated to" Comment: personnel were aware of allocation. Investigator‐assessed outcomes |
| Blinding of outcome assessment (detection bias) Objective outcome | Low risk | Quote from publication: "... research assistants [measuring weight and height] were blinded to the research condition." |
| Incomplete outcome data (attrition bias) Objective outcomes | Unclear risk | Comment: 2 clusters (1 per arm) not analysed. Also states used ITT, numbers analysed for BMI and waist circumference outcomes differ, assume because of ICC for clustering |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes stated are reported and response from study author concurs |
| Other bias | Unclear risk | Comment: 211 parent‐child dyads in the intervention group did not receive any of the counselling sessions |
Small 2013.
| Methods |
Parallel randomised controlled trial Randomisation ratio: not reported Superiority design |
|
| Participants |
Inclusion criteria: parents of overweight or obese 4‐ to 8‐year‐old Exclusion criteria: children who had uncontrolled medical problems (e.g. asthma) that might preclude them from fully participating in the intervention Diagnostic criteria: described only as overweight or obese |
|
| Interventions |
Number of study centres: unclear Treatment before study: none Titration period: none Description of interventions Before each of the sessions for either group, parents were provided with age‐appropriate, audio‐taped, educational information on a range of topics. Each group had 4 face‐to‐face sessions held for 30‐60 minutes and 3 telephone calls between sessions (duration unclear) Parent intervention: Theoretically based intervention. Parents were offered educational information about the establishment of healthy habits in young children, nutritional information, information regarding increasing physical activity and decreasing sedentary time, and age‐specific information regarding the child's behaviour in response to change 4 face‐to‐face sessions during which trained research assistants used principles of brief motivational interviewing (i.e. elicit information from the participant, provide non‐judgemental information, and elicit the participant's understanding; to collaborate with parents on identifying specific realistic healthy lifestyle goals, developing clear steps to reach those goals, routinely having the parents re‐evaluate progress, and identifying new goals as needed) Parents were provided with specific feedback about their child (i.e. physical activity and dietary intake) before establishing goals for their child and family. All face‐to‐face parent intervention sessions were separated by 4‐6 weeks to provide each family with time to enact planned changes, encounter child responses to those changes and review new educational information before the next face‐to‐face session Telephone calls were made to each parent to:
At each of the 4 measurement time points, parents were offered USD 35 as remuneration for their time in completing the various measurements. Each child was given a group specific (e.g. treatment group and control group) bag of toys to facilitate activities that parents would be encouraged to complete with their child Sessions taken by trained research assistants who were also supervised Control: Parents were provided with educational age‐appropriate, evidence‐based health and safety information (e.g. care for thermal injuries, first‐aid care, and care for insect bites and stings) that is specific to parenting in the southwest US. Parents met with a control interventionist and in a similar way were encouraged to make health and safety goals for their family (e.g. development of first‐aid materials and identification of a fire escape plan) Telephone calls were made to each parent as described above |
|
| Outcomes | Outcomes reported in abstract of publication: waist circumference, waist‐by‐height ratio, BMI and BMI percentile | |
| Study details |
Run‐in period: none Study terminated before regular end (for benefit/because of adverse events): no |
|
| Publication details |
Language of publication: English Non‐commercial funding Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "The purpose of this randomised control pilot study was to determine the feasibility and preliminary effects of a theoretically based, primary care intervention on the physical outcomes of 60 overweight/obese preschool/early school‐aged 4‐ to 8‐year‐old children..." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from publication: "each parent‐child dyad was randomly assigned to the treatment or control condition..." Comment: no description provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: no description provided |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Comment: no details provided |
| Blinding of outcome assessment (detection bias) Objective outcome | Unclear risk | Comment: no details provided |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | Quote from publication: "Seven parent‐child dyads returned incomplete T1 data, and thus we removed data from those dyads from all other analyses. As a result, experimental and control group sample sizes were 33 and 27, respectively" Comment: multiple imputation techniques to account for the missing data in subsequent analyses for the remaining 60 parent‐child dyads |
| Selective reporting (reporting bias) | Unclear risk | Comment: all outcomes reported as stated but not enough information to judge |
| Other bias | Unclear risk | Comment: not enough detail to judge |
Esfarjani 2013.
| Methods |
Parallel randomised controlled trial Randomisation ratio: 1 : 1 Superiority design |
|
| Participants |
Inclusion criteria: aged 7 years, ≥ 95th percentile of BMI for age Exclusion criteria: mental retardation, psychiatric symptoms, current obesity treatment, chronic disease and use of medication Diagnostic criteria: obesity defined as BMI 95th percentile for age by 2000 reference standards |
|
| Interventions |
Number of study centres: 1 Treatment before study: none Titration period: none Intervention description: Parent‐only: 12 session training programme over 6 months 8 weekly sessions for the first 2 months, then 4 monthly sessions. Each session lasts 4 hours, including a review of parent progress in implementing strategies developed for changing child's eating or exercise habits, and the specific topic of the day, such as learning about the reasons of the childhood obesity, receiving nutritional information (e.g. food pyramid, food choices, food labels, food preparation and cooking, eating habits, regular meals, controlling environments that stimulate overeating, special dietary consideration during holidays and at the restaurants) and guidelines for physical activity and reducing sedentary behaviours (e.g. reduce watching TV and playing computer games, use stairs instead of lifts and play outside instead of inside) Control: 2 sessions of training programme (occurred after intervention group's 6‐month training programme), no details provided |
|
| Outcomes | Outcomes reported in abstract of publication: weight, waist and hip circumference, cholesterol, serum triglycerides, food group consumption, TV and computer time, walking time | |
| Study details |
Run‐in period: none Study terminated before regular end (for benefit/because of adverse events): no |
|
| Publication details |
Language of publication: English Non‐commercial funding Peer review publication |
|
| Stated aim for study | Quote from publication: "to assess the effect of lifestyle modification family‐based intervention in young Iranian children" | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from publication: "children got a code number and were randomly divided into two groups" Comment: randomly assigned |
| Allocation concealment (selection bias) | Unclear risk | Comment: not enough information to judge |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Comment: adjudicated/investigator‐assessed, no other details |
| Blinding of outcome assessment (detection bias) Objective outcome | Unclear risk | Comment: no details provided |
| Incomplete outcome data (attrition bias) Objective outcomes | High risk | Comment: numbers only reported, numbers in flow chart and results do not match, differential drop‐out rates |
| Selective reporting (reporting bias) | Unclear risk | Comment: all outcomes reported as stated but not enough information to judge |
| Other bias | Unclear risk | Comment: not enough information to judge |
Moens 2012.
| Methods |
Parallel randomised controlled trial Randomisation ratio: not reported Superiority design |
|
| Participants |
Inclusion criteria: child aged 6‐12 years, 20‐85% overweight, medical clearance form a physician Exclusion criteria: secondary overweight caused by endocrinological, chromosomal or hypothalamic disease or mental retardation Diagnostic criteria: as above |
|
| Interventions |
Number of study centres: 1 Treatment before study: none Titration period: none Intervention description: Parent‐only intervention: 6 group meetings of 2 hours each over a 5‐month period. Provided information with a focus on weight control not weight loss to re‐establish a sense of healthy balance between energy intake and energy expenditure. Parent workbook. Education on different food groups, detailed product information and child‐friendly recipes. Used the Food Pyramid. Parenting skills focused on understanding of eating habits and lifestyles, cognitive and behavioural barriers to change, general parenting skills of positive involvement, monitoring, problem‐solving skills and maintaining positive changes already effected; self control and healthier lifestyle sessions (full details of content provided) Sessions conducted by dietician and a psychologist under supervision of a behavioural therapist and a manual for each session was available Control: Waiting list control |
|
| Outcomes | Outcomes reported in abstract of publication: BMI, parental report of child's eating behaviour, familial health principles | |
| Study details |
Run‐in period: none Study terminated before regular end (for benefit/because of adverse events): no |
|
| Publication details |
Language of publication: English Commercial funding Peer review publication |
|
| Stated aim for study | Quote from publication "To evaluate a parent‐led intervention for overweight children between 6 and 12 years old" | |
| Notes | Also report a follow‐up study comparing all families post intervention with a sample of families who did not respond to the original invitation. Not relevant here | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote from publication: "randomly assigned on the basis of the date on which they contacted the research group" Comment: high risk |
| Allocation concealment (selection bias) | High risk | Comment: assignment by a co‐worker, no other details |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Quote from study author: "Participants knew whether they would start immediately (intervention group); or had to wait (waitlist condition)" Comment: no details blinding of study personnel |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Quote from study author: "Participants knew whether they would start immediately (intervention group); or had to wait (waitlist condition)" Comment: no details blinding of study personnel |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Comment: no details, as height and weight considered by reviewers as a subjective outcome as were obtained by parental report |
| Blinding of outcome assessment (detection bias) Objective outcome | High risk | Quote from study author: "height and weight were obtained by parental report" |
| Incomplete outcome data (attrition bias) Subjective outcomes | Unclear risk | Comment: questionnaire completion rates provided and some withdrawn by study authors as missing items |
| Incomplete outcome data (attrition bias) Objective outcomes | Unclear risk | Comment: reports numbers but not reasons for drop‐out. All drop‐outs from waiting list control group so some imbalance. No description of how analysed |
| Selective reporting (reporting bias) | High risk | Comment: subjective outcomes not reported at baseline or follow‐up for the 2 groups separately but are reported for both combined |
| Other bias | Unclear risk | Comment: is reported to be a pilot study so unclear if sample size is appropriate to detect a difference |
Raynor 2012a.
| Methods |
Parallel randomised controlled trial Randomisation ratio: 1 : 1 : 1 Superiority design |
|
| Participants |
Inclusion criteria: ≥ 85th percentile for BMI as determined by the Centers for Disease Control growth charts, having no dietary or physical activity restrictions Exclusion criteria: participating parent could not read English, had a psychological disorder that would impair ability to participate or if the family was planning to move out of the area during the programme Diagnostic criteria: BMI ≥ 85th percentile |
|
| Interventions |
Number of study centres: not reported Treatment before study: none Titration period: none Description of interventions Behavioural parent‐only intervention: 8 sessions, 45 minutes each Focused on increasing child growth monitoring and providing feedback to families 2 interventions that combined the parent‐only intervention with a 6‐month, behavioural, parent‐only intervention that focused on 2 energy‐balance (diet) behaviours: [DECREASE] Decreasing sugar sweetened beverage and sweet and salty snack food intake. Children and parents reduced intake of sweet and salty snack foods (i.e. candy, cookies, ice cream, chips, nuts) to ≤ 3 servings/week, and sugar sweetened beverages (i.e. soda, Kool‐aid, sweetened tea, non‐100% fruit juice, sports drinks) to ≤ 3 servings/week [INCREASE] Increasing fruit, vegetable and low‐fat dairy intake. Children and parents were encouraged to consume 2 servings/day of whole fruit, 3 servings/day of vegetables and 2 servings/day of low‐fat dairy products Sessions covered behavioural lessons and emphasised monitoring of targeted behaviours, pre‐planning, problem solving, shaping, setting goals, positive reinforcement, stimulus control and parental modelling of targeted behaviours. These behavioural strategies are endorsed in both the 1997 and 2007 recommendations. Children and their parents self monitored the targeted behaviours and turned in records at each meeting Families received USD 20 for completing each of the 6‐ and 12‐month assessments Based on behavioural economics theory (changing a substitute behaviour of a target behaviour enhances the feeling of choice for engaging in and liking the targeted behaviour, which could increase long‐term adherence). Meetings were led by an experienced research‐staff therapist (either master or doctoral level) with expertise in nutrition or exercise science, and behaviour modification Following the 6‐month intervention, all families received feedback on growth at 9 months, and final assessments were conducted at 12 months |
|
| Outcomes | Outcomes reported in abstract of publication: BMI z score, energy intake | |
| Study details |
Run‐in period: none Study terminated before regular end (for benefit/because of adverse events): no |
|
| Publication details |
Language of publication: English Non‐commercial funding Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "to examine the efficacy of U.S. primary care paediatric obesity treatment recommendations, within two randomised trials" | |
| Notes | Authors provided change data from baseline to immediate and longest follow‐up for BMI z score, following contact to request further data. There are 2 comparisons of relevance to this review, the parent‐only vs. the decrease group and the parent‐only vs. the increase group |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: "Participants in each trial were randomly assigned to one of three interventions in a 1:1:1 allocation ratio. Using random permuted blocks within strata (gender), cards with intervention assignment were sealed in an envelope by research staff not engaged in intervention or assessments and provided to families at a randomisation visit, following completion of baseline assessments" |
| Allocation concealment (selection bias) | Low risk | Quote from publication: "Participants in each trial were randomly assigned to one of three interventions in a 1:1:1 allocation ratio. Using random permuted blocks within strata (gender), cards with intervention assignment were sealed in an envelope by research staff not engaged in intervention or assessments and provided to families at a randomisation visit, following completion of baseline assessments" |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Comment: masking of carers and participants to allocation but unclear if blinded to intervention |
| Blinding of outcome assessment (detection bias) Objective outcome | Low risk | Quote from publication: "Dependent measures were collected by trained research‐staff blinded to treatment assignment" Comment: outcome assessors masked to treatment assignment |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | Comment: ITT analysis. Missing data filled using a multiple imputation strategy. Specifically, for each participant with a missing BMI z score value, 5 random variables from a normal distribution that has a mean equal to the baseline BMI z score and variance equal to the estimated variance for BMI z score of other participants at the time where BMI z score is missing |
| Selective reporting (reporting bias) | Unclear risk | Comment: all outcomes reported as stated but not enough information to judge |
| Other bias | Unclear risk | Comment: not enough detail to judge |
Raynor 2012b.
| Methods |
Parallel randomised controlled trial Randomisation ratio: 1 : 1 : 1 Superiority design |
|
| Participants |
Inclusion criteria: ≥ 85th percentile for BMI as determined by the Centers for Disease Control growth charts, having no dietary or physical activity restrictions Exclusion criteria: participating parent could not read English, had a psychological disorder that would impair ability to participate or if the family was planning to move out of the area during the programme Diagnostic criteria: BMI ≥ 85th percentile |
|
| Interventions |
Number of study centres: not reported Treatment before study: none Titration period: none Description of interventions Behavioural parent‐only intervention: as above. 2 additional interventions were: [TRADITIONAL] Decreasing sugar‐sweetened beverage intake and increasing physical activity. Encouraged children to reach 60 minutes/day (parents 30 minutes/day) of moderate‐intensity physical activity most days of the week and for children and parents to consume ≤ 3 servings of sugar‐sweetened beverages/week. [SUBSTITUTE] Increasing low‐fat milk intake and decreasing TV watching, encouraged children and parents to watch ≤ 2 hours of TV/day and to consume 2 servings of low‐fat milk/day |
|
| Outcomes | Outcomes reported in abstract of publication: BMI z score, energy intake | |
| Study details |
Run‐in period: none Study terminated before regular end (for benefit/because of adverse events): no |
|
| Publication details |
Language of publication: English Non‐commercial funding Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "to examine the efficacy of U.S. primary care paediatric obesity treatment recommendations, within two randomised trials" | |
| Notes | There are 2 comparisons of relevance to this review: the parent‐only vs. the substitute group and the parent‐only vs. the increase group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: "Participants in each trial were randomly assigned to one of three interventions in a 1:1:1 allocation ratio. Using random permuted blocks within strata (gender), cards with intervention assignment were sealed in an envelope by research staff not engaged in intervention or assessments and provided to families at a randomisation visit, following completion of baseline assessments" |
| Allocation concealment (selection bias) | Low risk | Quote from publication: "Participants in each trial were randomly assigned to one of three interventions in a 1:1:1 allocation ratio. Using random permuted blocks within strata (gender), cards with intervention assignment were sealed in an envelope by research staff not engaged in intervention or assessments and provided to families at a randomisation visit, following completion of baseline assessments..." |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Comment: masking of carers and participants to allocation but unclear if blinded to intervention |
| Blinding of outcome assessment (detection bias) Objective outcome | Low risk | Quote from publication: "Dependent measures were collected by trained research‐staff blinded to treatment assignment.." Comment: outcome assessors masked to treatment assignment |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | Comment: ITT analysis. Missing data filled using a multiple imputation strategy. Specifically, for each participant with a missing BMI z score value, 5 random variables from a normal distribution that has a mean equal to the baseline BMI z score and variance equal to the estimated variance for BMI z score of other participants at the time where BMI z score is missing |
| Selective reporting (reporting bias) | Unclear risk | Comment: all outcomes reported as stated but not enough information to judge |
| Other bias | Unclear risk | Comment: not enough detail to judge |
Magarey 2011.
| Methods |
Parallel randomised controlled trial Randomisation ratio: not reported Superiority design |
|
| Participants |
Inclusion criteria: aged 5.0‐9.9 years, overweight (International Obesity Task Force definition) and prepubertal (Tanner stage 1), having a carer willing to attend sessions and able to speak English Exclusion criteria: BMI z score of 4.0, having a syndromal cause of obesity, using medications that influence weight, having a physical or developmental disability, having a chronic illness or having a sibling enrolled in the study Diagnostic criteria: overweight (International Obesity Task Force definition) |
|
| Interventions |
Number of study centres: 3 Treatment before study: none Titration period: none Description of interventions 24‐week intervention delivered by a dietician (accredited training for the parenting skills component) 2 groups: parent healthy lifestyle group and healthy lifestyle group The interventions included 12 (parent group) or 8 (healthy lifestyle) 90‐ to 120‐minute group sessions (open to both parents but mostly attended by mothers) and 4 telephone sessions, delivered over 6 months with tapered frequency (weekly, bimonthly, then monthly). Details of the standardised parenting skills programme and the healthy lifestyle education sessions reported (references given). The 4 telephone sessions alternated with the last 4 group sessions for both arms, using a standard protocol Parenting healthy lifestyle: The Positive Parenting Program (Triple P) was delivered in 4 sessions before the healthy lifestyle component. It is a standardised and evaluated generic parenting programme widely used in Australia and provides comprehensive facilitator training Healthy lifestyle: The 8 sessions included recommendations on specific core food servings; practical skills for healthy eating, reduced sedentary behaviours and increased activity; and monitoring of lifestyle behaviours and roles and responsibilities around eating, managing appetite, self esteem and teasing. Children and siblings participated in fun, non‐competitive activity sessions run by physical activity educators. These sessions provided optional active child care for participants in both groups and were not part of the intervention |
|
| Outcomes | Outcomes reported in abstract of publication: BMI z score, waist z score | |
| Study details |
Run‐in period: no clear run‐in period Study terminated before regular end (for benefit/because of adverse events): no |
|
| Publication details |
Language of publication: English Non‐commercial funding Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "... to evaluate (1) the effectiveness of a healthy lifestyle intervention for overweight children aged 5 to 9 years that targets parents as the agents of change and (2) whether additional specific parenting skills training would improve parenting skills and enhance the intervention effect. The long‐term effect (2 years from baseline) and the immediate postintervention effect (at completion of the intervention, 6 months from baseline) were assessed. We also aimed to confirm gender differences reported in our previous study..." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: ".. After baseline measurements, participants were allocated to intervention groups using computer‐generated randomisation schedules stratified according to gender and recruitment site and prepared by staff not otherwise involved in the study" |
| Allocation concealment (selection bias) | Low risk | Quote from publication: "Allocation was concealed in opaque, sequentially numbered, sealed envelopes and opened by parents after completion of baseline measurements..." |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Comment: a single‐blinded randomised controlled trial blind at point of allocation, but not blinded to which intervention they received |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Comment: a single‐blinded randomised controlled trial blind at point of allocation, but not blinded to which intervention they received |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Comment: those who took measurements were blinded to type of intervention |
| Blinding of outcome assessment (detection bias) Objective outcome | Low risk | Comment: those who took measurements were blinded to type of intervention |
| Incomplete outcome data (attrition bias) Subjective outcomes | High risk | Comment: stated ITT was conducted using all available data according to allocation, regardless of attendance (details provided). However, a second per‐protocol analysis was performed that included only those who attended ≥ 75% of the programme sessions. The potential effect of missing data were explored using T tests to compare the baseline and 6‐month BMI z scores of those who remained and those who were lost to follow‐up. No reasons for drop‐outs were given. It is unclear whether data presented were ITT or not |
| Incomplete outcome data (attrition bias) Objective outcomes | High risk | Comment: stated ITT was conducted using all available data according to allocation, regardless of attendance (details provided). However, a second per‐protocol analysis was performed that included only those who attended ≥ 75% of the programme sessions. The potential effect of missing data were explored using T tests to compare the baseline and 6‐month BMI z scores of those who remained and those who were lost to follow‐up. No reasons for drop‐outs were given. It is unclear whether data presented were ITT or not |
| Selective reporting (reporting bias) | High risk | Comment: states health‐related quality of life measured but no data reported |
| Other bias | Unclear risk | Comment: no true control group. Parenting outcomes only given for the whole sample, not split into intervention and control groups ‐ therefore success of intervention in each group cannot be compared. Retention rates moderate for the long follow‐up period. Unclear if ITT analysis was performed. Likely the participants who dropped out the study were more overweight |
Jansen 2011.
| Methods |
Parallel randomised controlled trial Randomisation ratio: not reported Superiority design |
|
| Participants |
Inclusion criteria: parents participated in the treatment voluntarily. Children's percentage of overweight had to be at least 130% Exclusion criteria: none stated Diagnostic criteria: overweight (as above) |
|
| Interventions |
Number of study centres: 3 Treatment before study: none Titration period: none Description of interventions: Parental CBT: 'Finger in the pie' was a cognitive behavioural treatment and each session addressed a different theme associated with childhood overweight. The purpose was not purely to present information, but to teach parents to think of alternatives and possible solutions themselves. This way, future coping abilities were addressed. The following themes were included: creating realistic expectations concerning the development of their children's weight status, modifying eating and exercising habits, knowledge on how parents can influence the behaviour of their children (e.g. by modelling and by the use of control and rewards), information on the development of overweight, handling feelings of guilt, and recognising and handling a child with low self esteem. So, instead of purely focusing on nutrition and physical activity, a substantial part of the treatment was devoted to enhancing parenting tactics (e.g. teaching parents to ignore undesirable behaviours and to reward desirable behaviours). This aspect of the treatment combined with extensively discussing parental control makes the current intervention distinguishing Of the 2‐hour sessions, the first hour was interactive. 1 of the main goals of this first hour was to identify wrong thought patterns and challenging these patterns. The second hour of each session was more informative and practical by nature Behavioural and nutritional components Session 1 ‐ the part that parents play; session 2 ‐ eating behaviour; session 3 ‐ physical exercise; session 4 ‐ parental control; session 5 ‐ be in good spirit; session 6 ‐ food and party; session 7 ‐ relapse; session 8 ‐ responsibility Intervention carried out according to a protocol, written by the first and the second authors and carried out by trained cognitive behavioural therapists Waiting list control: Offered the treatment after 6 months |
|
| Outcomes | Outcomes reported in abstract of publication: BMI percentile, relapse, psychopathology, self esteem and negative thoughts | |
| Study details |
Run‐in period: none Study terminated before regular end (for benefit/because of adverse events): no |
|
| Publication details |
Language of publication: English Funding not stated Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "The aim of the current study is to investigate whether a treatment that aims at parents exclusively (by targeting eating and exercise behaviours, and supporting self‐esteem of the child in a cognitive‐behavioural manner) would be successful in reducing their children's overweight. Such a treatment might lead to better results than treatments focusing on children, as parents play an important role in their children's eating and exercising behaviours, and in promoting their self‐esteem..." | |
| Notes | Randomisation was broken because 9 participant families from the waiting list control were included in the intervention arm, the study did not report the numbers randomised to each group, just the total numbers randomised, the group sizes were unbalanced (59 vs. 39), and the paper states that 9 were 'included in the treatment group' | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from publication: "Parents were randomly assigned to either the treatment group or the waiting‐list control group" Comment: no description of randomisation process |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details provided |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Unclear risk | Comment: no details provided |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Comment: no details provided |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Comment: no details provided |
| Blinding of outcome assessment (detection bias) Objective outcome | Unclear risk | Comment: no details provided |
| Incomplete outcome data (attrition bias) Subjective outcomes | Low risk | Comment: no details provided |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | Comment: ITT analysis. The missing values at the follow‐up meeting were replaced by the last‐observation‐carried‐forward. The missing in‐between values (post‐treatment) were replaced by the mean of the values before treatment and at 3‐month follow‐up. Numbers of drop‐outs reported |
| Selective reporting (reporting bias) | Unclear risk | Comment: all outcomes reported as stated but not enough information to judge |
| Other bias | High risk | Comment: as only 9 of the 48 families in the waiting‐list control group eventually decided to participate in the treatment after the waiting period, it was decided to include these 9 families in the treatment group, and to disregard their data from the waiting period. These 9 families did not differ from the original treatment group or the control group |
Collins 2011.
| Methods |
Parallel randomised controlled trial Randomisation ratio: not reported Superiority design |
|
| Participants |
Inclusion criteria: overweight or obese (defined according to age‐ and sex‐specific international BMI cut‐off points (reference provided); aged 5‐9 years; pre‐pubertal (no pubic hair ‐ Tanner stage 1); generally healthy Exclusion criteria: extreme obesity (BMI z score > 4); known syndromal cause of obesity; long‐term steroid use; medications associated with weight gain; chronic illness; significant dietary restrictions Diagnostic criteria: overweight or obese (defined according to age‐ and sex‐specific international BMI cut‐off points |
|
| Interventions |
Number of study centres: not reported Treatment before study: not reported Titration period: not reported Detailed description of all interventions Parent DIET Intervention: Aimed at parents, not children. Informs parents of how to improve their child's diet. Based on the "Health Belief Model" (reference provided). 10 parent focused face‐to‐face group sessions ‐ each 2 hours long. 3 monthly telephone calls for the first 3 months of the intervention. Goal setting, problem‐solving, role‐modelling and positive reinforcement are used to manage changes in food behaviours and strategies incorporated to help parents increase their confidence in making changes related to their goals. The structure and content of the programme uses a cognitive behavioural, solution‐focused approach from the emerging field of health coaching. Behavioural change targeted during the sessions to reduce total energy and fat intakes, increase fruit and vegetable intake and make healthy beverage and snack choices. Practical advice about food shopping and preparation is provided with the sessions including a didactic component, group work and practical activities Delivered by a dietician Parent and child physical activity (child was main focus): Parent encouraged to set realistic short‐ to medium‐term SMART goals for increasing physical activity and reducing sedentary behaviours. Asked to identify barriers in their family lives that may prevent their child from participating in sufficient physical activity or that leads to their child spending excessive amounts of time in small screen recreation Children attended 10 x 2‐hour face‐to‐face weekly sessions. Each week children participate in a variety of activities aimed at improving their mastery of 12 fundamental movement skills (run, jump, leap, hop, slide, gallop, strike, roll, kick, throw, catch, bounce). Each session covered 3 fundamental movement skills, such that over the course of the 10 weeks each skill is re‐visited, although the focus is on more complex components of the skill, in subsequent sessions. Skill mastery is aided by adherence to lesson plans for each skill incorporating several learning stages:
1 Refresher session (2 hours) attended at week 18; 3 monthly telephone calls at weeks 14, 18 and 22 of the invention To maximise the children's competence and confidence, they were strongly encouraged to practice the fundamental movement skills at home with their parents or siblings (or both), between each group session. Each participant given a 'Home‐challenge folder', which included fun, relevant and developmentally appropriate activities enabling practice of skills at home. The home challenges took approximately 30 minutes and children were encouraged to complete 3 sessions each week Based on the "Competence Motivation Theory" (reference provided). Delivered by physical education teachers Parent‐Child Physical Activity + DIET: Same components as the DIET and physical activity interventions. 25 sessions. Delivered by physical education teachers and dieticians. 10 Parent‐focused face‐to‐face group sessions ‐ each were 2 hours long (10 weeks); 10 child‐focused face‐to‐face group sessions ‐ each 2 hours long (10 weeks); 1 refresher session (2 hours) attended at week 18; 3 monthly telephone calls at weeks 14, 18 and 22 of the invention; 1 parent workshop ‐ 1 hour long |
|
| Outcomes | Outcomes reported in abstract of publication: BMI z score, waist measurements, metabolic outcomes | |
| Study details |
Run‐in period: not reported Study terminated before regular end (for benefit/because of adverse events): no |
|
| Publication details |
Language of publication: English Non‐commercial funding Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "Outcomes of childhood obesity interventions are rarely reported beyond 1 year. We hypothesized that the impact on the BMI z score from a child‐centred physical‐activity program in combination with a parent‐entered dietary‐modification program would be greater than either program conducted alone at 24 months' after baseline" | |
| Notes | There were 2 comparisons of relevance to this review: the parent‐only vs. parent‐child physical activity group and the parent‐only vs. the parent‐child physical activity and diet group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: "To randomly allocate participants to one of the three intervention groups the bias coin method of allocation, using a computer‐based random number‐producing algorithm, is used. This method ensures an equal chance of allocation to each group. Stratification by gender and site is done to ensure an equal representation in groups at each site. Only one study member at each site has access to the allocation codes and these are stored on a password‐protected computer" |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details provided |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Comment: no blinding |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Comment: no blinding |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Quote from publication: "Outcome measures were assessed at baseline and 6, 12, and 24 months by trained assessors blinded to group assignment..." |
| Blinding of outcome assessment (detection bias) Objective outcome | Low risk | Comment: "Outcome measures were assessed at baseline and 6, 12, and 24 months by trained assessors blinded to group assignment..." |
| Incomplete outcome data (attrition bias) Subjective outcomes | High risk | Comment: ITT performed, however, high numbers not completing (35% to 51% completers after 2 years) |
| Incomplete outcome data (attrition bias) Objective outcomes | High risk | Comment: ITT performed, however, high numbers not completing (35% to 51% completers after 2 years) |
| Selective reporting (reporting bias) | High risk | Comment: only the 24‐month results are shown |
| Other bias | Unclear risk | Comment: not enough information to judge |
Boutelle 2011.
| Methods |
Parallel randomised controlled trial Randomisation ratio: not reported Non‐inferiority design: 1‐sided confidence interval |
|
| Participants |
Inclusion criteria: parents and their overweight or obese (> 85th BMI percentile) children, aged 8‐12 years. At least 1 parent or guardian participated with the child. If 2 children in the family met criteria for the study, both were invited to attend the treatment groups but a coin‐flip was used to determine which child's data would be part of the study Exclusion criteria: either the child or parent currently involved in psychological or weight loss treatment, using medications that affected weight or appetite, or had a psychiatric or physical condition (e.g. eating disorder, psychosis) that would interfere with participation Diagnostic criteria: as above (> 85th BMI percentile) |
|
| Interventions |
Number of study centres: 2 Treatment before study: none Titration period: none Intervention description: Parent‐only: Behavioural change skills; included self monitoring of targeted behaviours, positive reinforcement, stimulus control, pre‐planning and modelling. Parents in the parent‐only group were coached on how to assist their children in weight monitoring at home and reflect on the behaviours that influenced weight. Goal setting for the parent‐only group occurred during the treatment groups. Completed quizzes each week to assure knowledge of the treatment protocol. The intervention was theoretically based: using a current state‐of‐the‐art behavioural treatment for childhood obesity described by Epstein and colleagues (references provided) All interventionists attended a 3‐day training regarding the behavioural intervention for the study, and were supervised by the first author on a weekly basis during treatment. The intervention was provided by a psychologist Parent‐child intervention: The material taught in the child groups was similar in content to that taught to the parents and described above, but presented in an age‐appropriate manner (i.e. fun games). Parent‐child dyads also met with an interventionist either pre‐ or post group for family goal setting for a maximum of 10 minutes. All parents and children completed quizzes each week to assure knowledge of the treatment protocol. Components of the interventions in common between the groups included:
|
|
| Outcomes | Outcomes reported in abstract of publication: inferiority of treatment group on child weight loss, parent weight loss and child physical activity, caloric intake | |
| Study details |
Run‐in period: none Study terminated before regular end (for benefit/because of adverse events): no |
|
| Publication details |
Language of publication: English Non‐commercial funding Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "to evaluate whether a standardized behavioral parent only treatment program would not be inferior to a standardized behavioral parent‐child program on child weight loss and other relevant markers of change" | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: 80 parent‐child dyads were randomly assigned to either parent‐child or parent‐only groups. Random assignment was conducted after completing the initial assessment using a computer‐generated random numbers table |
| Allocation concealment (selection bias) | Unclear risk | Comment: no further details |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Comment: no details provided |
| Blinding of outcome assessment (detection bias) Objective outcome | Unclear risk | Comment: no details provided |
| Incomplete outcome data (attrition bias) Objective outcomes | High risk | Comment: ITT analysis but data not presented. A multiple imputation approach was used as a form of sensitivity analysis, which yielded non‐substantive differences with full‐likelihood approach to analysing the data that are reported. Parent‐only: 24/40 pairs completed follow‐up. Parent‐child: 28/40 pairs completed follow‐up. No further details |
| Selective reporting (reporting bias) | Unclear risk | Comment: all outcomes reported as stated but not enough information to judge |
| Other bias | Unclear risk | Comment: not enough information to judge |
West 2010.
| Methods |
Parallel randomised controlled trial Randomisation ratio: not reported Superiority design |
|
| Participants |
Inclusion criteria: parent described the child's body size as overweight, the child was 4‐11 years of age, and the parent agreed to attend a 12‐week intervention Exclusion criteria: child taking medication that affected growth or weight control, or had a severe developmental delay or disability Diagnostic criteria: parental description of child as overweight |
|
| Interventions |
Number of study centres: 6 Treatment before study: none Titration period: none Description of the intervention: Parent‐only: 12‐week intervention, 1 session per week. Group Lifestyle Triple P is a modification of Level 4 Group Triple P tailored to the concerns of parents of overweight and obese children. The intervention is a 12‐week intervention that consists of 9 x 90‐minute group sessions and 3 x 20‐minute telephone sessions. To help parents acquire new knowledge and skills, all sessions used an active skills training process (e.g. demonstrating and rehearsing skills) within a self regulation framework (e.g. self selecting goals and self evaluating progress). Each parent received a workbook summarising the session content, and suggested between‐session tasks Group sessions: in the first group session, motivational interviewing techniques were used to enhance parents' commitment to change. During subsequent group sessions, a range of specific strategies were introduced and practised. These included: positive parenting strategies (e.g. keeping track of children's lifestyle behaviour, setting clear guidelines about food and activity, reinforcing healthy behaviour). Also physical activity strategies (e.g. reducing TV and computer time, increasing energetic play, encouraging involvement in sport) and nutrition strategies (e.g. establishing eating routines, modifying recipes, reading food labels) were introduced and practised Telephone sessions: during the telephone sessions, the facilitator reviewed parents' implementation of strategies, and problem‐solved any difficulties. The final group session covered progress review and maintenance of treatment gains Triple P programme was standardised. All sessions were facilitated by a clinical psychologist and accredited provider of Group Triple P (who co‐authored the intervention materials), with assistance from graduate students in nutrition and dietetics, physical education and psychology Control: Waiting list control for 12 weeks. Included a physical activity and nutritional advice components |
|
| Outcomes | Outcomes reported in abstract of publication: child BMI z score and weight‐related problem behaviour, confidence in managing children's weight‐related behaviour | |
| Study details |
Run‐in period: none Study terminated before regular end (for benefit/because of adverse events): no |
|
| Publication details |
Language of publication: English Non‐commercial funding Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "..to evaluate the effects of the intervention on parenting and child weight‐related behaviour, relative to a waiting list control condition. This study describes the evaluation of a lifestyle‐specific parenting program (Group Lifestyle Triple P) on multiple child and parent outcomes..." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: "Families were randomly allocated to either the intervention...or the waiting list control...Groups were allocated to conditions according to a list of computer generated random numbers..." |
| Allocation concealment (selection bias) | High risk | Quote from publication: "Allocation concealment and blinded outcome assessment were not possible due to limited staff and resources" Comment: no masking of allocation to intervention or control |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Comment: no masking |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Comment: no masking |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Comment: no masking |
| Blinding of outcome assessment (detection bias) Objective outcome | High risk | Comment: no masking |
| Incomplete outcome data (attrition bias) Subjective outcomes | Low risk | Comment: numbers missing reported and reasons explained; ITT analysis with the last point‐carried‐forward |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | Comment: numbers missing reported and reasons explained; ITT analysis with the last point‐carried‐forward |
| Selective reporting (reporting bias) | Unclear risk | Comment: all outcomes reported as stated but not enough information to judge |
| Other bias | Unclear risk | Comment: not enough information to judge |
Resnick 2009.
| Methods |
Parallel randomised controlled trial Randomisation ratio: not reported Superiority design |
|
| Participants |
Inclusion criteria: eligible parents must have had a child: (a) with a BMI ≥ 85th percentile, (b) enrolled at 1 of the 2 study schools, (c) between Grades K and 5, (d) parents also had to agree to be randomised to either 1 of the 2 study conditions. In instances when families had multiple children enrolled at a study school with BMIs ≥ 85th percentile, the oldest child was considered the index child Exclusion criteria: none stated Diagnostic criteria: 85th percentile BMI |
|
| Interventions |
Number of study centres: 2 Treatment before study: none Titration period: none Description of interventions: Focus groups with 9 parents helped to determine study content, messages, and potential use of the 2 delivery methods, 1‐to‐1 counselling and mailed materials Educational mailed materials: Posted approximately every 5 weeks, 6 mailings over 30 weeks The educational material included: tips to increase walking, talk with children about TV viewing. Received a physical activity book and a pedometer. Read nutrition labels, shop more healthfully at grocery stores, talk with children about eat out healthfully. Received a cookbook, had a hands‐on activity about portion sizes Educational material plus personal encounters: Received the same educational materials as above The type of encounter (home visit, telephone call, etc.) was based on participating parents' preferences and schedules. Parents selected the topics discussed during each visit from choices provided by community health workers (CHWs) (i.e. epidemiology of childhood overweight, biological, social and environmental influences on childhood overweight; basic nutrition; label reading; grocery shopping strategies, including a tour of a local grocery store; physical activity guidelines) Mean 3.4 personal encounters over the 30 weeks. On average, parents received encounters for 18 weeks Both interventions were delivered by CHWs who attended a 36‐hour training programme over the course of 6 days. The purpose of the training was to prepare CHWs to make evidence‐based recommendations to families such as changing to reduced fat milk, reducing the intake of snack foods, replacing sugar‐sweetened beverages with water, increasing fruit and vegetable intake, decreasing TV viewing and increasing physical activity. The topics covered during the training included guidelines for home visits; the epidemiology of childhood overweight, biological, social and environmental influences on childhood overweight; basic nutrition; label reading; grocery shopping strategies, including a tour of a local grocery store; physical activity guidelines and counselling strategies. CHWs practiced counselling skills during their training by engaging in role‐plays with each other. After training and throughout the study, the study staff and CHWs met monthly to discuss specific concerns or difficulties with study participants |
|
| Outcomes | Outcomes reported in abstract of publication: BMI | |
| Study details |
Run‐in period: none Study terminated before regular end (for benefit/because of adverse events): no |
|
| Publication details |
Language of publication: English Non‐commercial funding Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "The goal was to create an easy‐to‐use parent outreach model that could ultimately be used by school nurses, paediatricians, community health agencies, and CHWs" | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from publication: "Parents from two schools were randomly selected to participate in one of two study groups and were randomised to either one of the two study conditions..." Comment: no description of randomisation process |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details provided |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Comment: no details provided |
| Blinding of outcome assessment (detection bias) Objective outcome | Unclear risk | Comment: no details provided |
| Incomplete outcome data (attrition bias) Objective outcomes | High risk | Comment: no ITT analysis. In all, 42/46 (91%) parents completed post‐intervention surveys |
| Selective reporting (reporting bias) | Unclear risk | Comment: all outcomes reported as stated but not enough information to judge |
| Other bias | Unclear risk | Comment: not enough detail to judge |
Estabrooks 2009.
| Methods |
Parallel randomised controlled trial Randomisation ratio: not reported Superiority design |
|
| Participants |
Inclusion criteria: BMI ≥ 85th percentile for their age Exclusion criteria: plans to move out of the state during the course of the study, a request by the child's paediatrician that the family not be contacted Diagnostic criteria: BMI ≥ 85th percentile for their age |
|
| Interventions |
Number of study centres: not reported Treatment before study: not reported Titration period: not reported Description of interventions: Based on social‐ecological theory (reference provided). 3 intervention groups: workbook, group sessions, group sessions + interactive voice response (IVR) counselling Parent group + IVR counselling: Group sessions as above. Then 10 follow‐up sessions by IVR, 1 per week. Included behavioural, physical activity and nutritional components (12‐week intervention) Physical activity get at least 2.5 hours of moderate physical activity this week; make a list of your family's barriers to physical activity around the house, and come up with a way to overcome them; do something active with your child for 15 minutes at least 3 times this week; review the family goal sheet with your family and set measurable, specific and objective goals this week; decrease your sitting time by 0.5 hours each day this week; increase your physical activity by 1 day per week; set a goal to take your family to a new park or trail that you have not visited before; survey your family regarding physical activity that they would like to do as a family, and try to do that activity at least 3 times per week Nutrition parent healthy habits: set a good example for your child by eating 5‐9 servings of fruits and vegetables every day this week; drink low‐fat milk at 1 meal each day; clear the kitchen cupboards of unhealthy snacks; prepare at least 1 healthy meal together with your child; post signs at least twice this week about your family's positive changes with healthy eating; decrease your soda and sugared‐drink consumption by 1 serving per day; increase your servings of fruits and vegetables by 3 per day; check your library for cookbooks, recipes or videos that help your family to prepare nutritious meals; change 1 food item that is high‐fat to a healthy snack of fruit or vegetable Behavioural consistency and contingency; communication; praise; parenting skills; support; plan; commitment Group sessions were led by a dietician (no further details) Parent group sessions: 2 group sessions. Utilised the workbook used in the control condition. Addressed parents' behavioural health skills and knowledge of weight, nutrition and physical activity. It also identified key parenting skills: limit setting, effective communication and role modelling. This session concluded with role playing, problem solving and the development of an action plan. 24‐week intervention Parent workbook group: 61‐page workbook to promote physical activity and the consumption of fruits and vegetables and decrease sugared‐drink consumption and TV viewing/recreational computer time. Activity to explore parental beliefs about eating and physical activity, healthy habits for creating a healthy family, defining the division of responsibility for eating and activity. Physical activity: using FITT (frequency, intensity, time, type) principles. Nutrition: helping children to avoid fad diets, reading labels, selecting healthy food options, sample menus, tips for preparing healthy snacks and meals. Assessing and calculating BMI in children and adults, causes of overweight in children (biological, cognitive, environmental), 5 reasons children gain weight, impact of being overweight, parenting skills to support weight reduction, survey of the family home environment, ways to promote a healthy home environment, goal setting: creating a family action plan, process of goal setting and keeping objectives clear, parent's personal action plan, barriers and strategies to maintaining family action plan. 24‐week intervention |
|
| Outcomes | Outcomes reported in abstract of publication: child BMI z scores, symptoms of eating disorders and body image | |
| Study details |
Run‐in period: not reported Study terminated before regular end (for benefit/because of adverse events): not reported |
|
| Publication details |
Language of publication: English Non‐commercial funding Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "A practical RCT [randomised controlled trial] evaluated the relative effectiveness of three interventions to support parents of overweight or at‐risk children to change the home environment to foster more healthful child eating and activity behaviours, thereby reducing child BMI and BMI z scores. A secondary purpose was to determine the patterns of use and potential dose effect for the highest‐intensity intervention" | |
| Notes | There were 3 comparisons of relevance to this review: the parent‐only group and IVR vs. the control (workbook); the parent‐only group vs. the control; the parent‐only group and IVR vs. the parent group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: "Through a random‐numbers table, participants were assigned randomly (families/staff unblinded) to the FC‐workbook, the FC‐group, or the FC‐IVR intervention..." |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Comment: families/staff were both unblinded |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Comment: families/staff were both unblinded |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Comment: families/staff were both unblinded |
| Blinding of outcome assessment (detection bias) Objective outcome | Low risk | Comment: families/staff were both unblinded, but probably no substantial impact on outcome measures |
| Incomplete outcome data (attrition bias) Subjective outcomes | Low risk | Comment: study says they carried out ITT analysis; however, results were only given for completers. 72% in workbook intervention, 66% in group intervention and 74% in group + IVR intervention completed 12‐month follow‐up ‐ drop‐outs rates average for this length of follow‐up |
| Incomplete outcome data (attrition bias) Objective outcomes | High risk | Comment: study says they carried out ITT analysis; however, results were only given for completers. 72% in workbook intervention, 66% in group intervention and 74% in group + IVR intervention completed 12‐month follow‐up ‐ drop‐out rates average for this length of follow‐up |
| Selective reporting (reporting bias) | High risk | Comment: states health‐related quality of life reported but no data reported |
| Other bias | Unclear risk | Comment: unsure if all the participants stayed in their randomised groups |
Munsch 2008.
| Methods |
Parallel randomised controlled trial Randomisation ratio: not reported Equivalence design |
|
| Participants |
Inclusion criteria: BMI > 85th percentile adjusted for gender and age. Participating family members were able to speak and write in German. All participants were free from diabetes, heart disease and endocrine disorders Exclusion criteria: parents and children meeting the criteria of the DSMIV‐TR for mental disorders warranting immediate treatment (assessed in a clinical interview), such as suicidal tendency, psychosis, mania, organic dementia or substance abuse disorder. Parents' or children's participation in a diet programme or other psychotherapy treatment with weight loss medication. There were only 4 fathers eligible for treatment, therefore, excluded from the analyses Diagnostic criteria: BMI > 85th percentile adjusted for gender and age |
|
| Interventions |
Number of study centres: 2 Treatment before study: none Titration period: no Description of interventions: the TAKE (Training adipöser Kinder und ihrer Eltern) programme. Programme overall proceeded with group sessions that began with a short overview of the topic, then individual difficulties and progress with homework were discussed and the group established coping strategies. Afterwards the major topics of the sessions were implemented and new homework was assigned. Interventions throughout the programme were highly interactive, proceeded step by step, and involved the group as a whole as well as individual mothers and children (where appropriate) Mother's CBT: 10 sessions in 5 phases over 10 weeks (120‐minute sessions) Phase 1 (nutrition and eating behaviour, covered across 8 sessions including psychoeducation about childhood obesity, risks, nutritional counselling, goal setting, stimulus control, family rules); phase 2 (physical activity, covered within 2 sessions including psychoeducation about physical activity, motivation, suitable sports); phase 3 (social competences, covered in 1 session, including social skills training, parental modelling and support of children dealing with difficulty situations); phase 4 (body concept, covered in 1 session, included supporting child's developments of a positive body concept); phase 5 (relapse prevention, covered in 1 session, included training of maintenance skills, appraisal of goal attainment, developing coping strategies). Children attended a relaxation training (progressive muscle relaxation training) of equal frequency and duration to the disorder‐specific CBT of children in the mother‐child group Mother‐child arm: 10 weekly treatment sessions of 120 minutes. Mothers received CBT including the same components as the Mothers‐only group. Children received sessions on nutrition and eating behaviour, basic nutritional education, reinforcement and tokens, lessons in physical activity, social competencies (self assertiveness, social skills, saying 'no' to food offers, role modelling, anti‐bullying plans), developing a positive body concept and relapse prevention Sessions were undertaken by psychologists and trained co‐therapists. All therapists were trained and supervised weekly by 1 of the authors |
|
| Outcomes | Outcomes reported in abstract of publication: per cent overweight, general behaviour problems (externalising and internalising behaviour problems), global and social anxiety, and depression | |
| Study details |
Run‐in period: none Study terminated before regular end (for benefit/because of adverse events): no |
|
| Publication details |
Language of publication: English Non‐commercial funding Publication status: peer‐reviewed journal and Word document |
|
| Stated aim for study | Quote from publication: "we investigated whether the treatment of parents only would be as efficacious as a parent‐child treatment in a randomised controlled clinical trial. Our group treatment approach, TAKE [Training adipöser Kinder und deren Eltern ('training of obese children and their parents')], targeted weight stabilization and reduction of behavioral problems of obese children aged 8‐12 years" | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from publication: "families were randomly assigned according to a permuted block design to either the mother‐child (condition A) or the mother‐only (condition B) cognitive behavioral therapy (CBT) treatment..." |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details provided |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Unclear risk | Comment: no details provided |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Comment: no details provided |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Comment: no details provided |
| Blinding of outcome assessment (detection bias) Objective outcome | Unclear risk | Comment: no details provided |
| Incomplete outcome data (attrition bias) Subjective outcomes | High risk | Comment: high rates of drop‐out (mother‐child 11/31 dropped out, in mother‐only 18/25 dropped out), no ITT analysis |
| Incomplete outcome data (attrition bias) Objective outcomes | High risk | Comment: high rates of drop‐out (mother‐child 11/31 dropped out, in mother‐only 18/25 dropped out), no ITT analysis |
| Selective reporting (reporting bias) | Unclear risk | Comment: all outcomes reported as stated but not enough information to judge |
| Other bias | Unclear risk | Comment: families were randomly assigned according to a permuted block design to either the mother‐child (condition A) or the mother‐only (condition B) CBT treatment. Selection bias: there were only 4 fathers eligible for treatment and they were excluded from the analyses. Also underpowered |
Janicke 2008.
| Methods |
Parallel randomised controlled trial Randomisation ratio: not reported Superiority design |
|
| Participants |
Inclusion criteria: BMI ≥ 85th percentile for age and gender, required to live within the same dwelling in a rural county that is designated in whole or in part as a "Health Professional Shortage Areas" by the U.S. Department of Health and Human Services, to obtain physician approval to participate in the study, with documentation provided via letter signed by a physician medically clearing individuals to participate in a weight management programme Exclusion criteria: child or participating parent has a dietary or exercise restriction, or a medical condition that contraindicates mild energy restriction or moderate physical activity (including a history of musculoskeletal condition that limits walking; heart condition; chronic lung diseases limiting physical activity; uncontrolled diabetes; uncontrolled hypertension; thyroid disease; or uncontrolled exercise‐induced asthma as determined by a physician). Children or participating parents on antipsychotic agents, systemic corticosteroids or who were currently using prescription weight‐loss drugs, insulin or other diabetic medications. Not engaged in another weight control programme, exhibit conditions or behaviours that were likely to affect their participation in the trial, such as being unwilling or unable to give informed consent, parent(s) or legal guardian(s) unable to read English at approximately the 5th grade level, unwilling to accept random assignment, unable to travel to extension office for intervention sessions, or likely to move out of the county within the next 12 months Diagnostic criteria: BMI ≥ 85th percentile for age and gender |
|
| Interventions |
Number of study centres: not reported Treatment before study: none Titration period: none Detailed description of interventions Behavioural parent‐based intervention: 12 sessions of 90 minutes Only the participating parent(s) attended group meetings. The primary treatment objectives were to build healthier dietary habits, increase moderate intensity physical activity, establish a healthier weight status and build positive self worth Each session included 3 segments, similar to the parent group for the Family‐Based intervention. An emphasis was placed on teaching parents how to work with their children to set goals. Each week interventionists suggested a range of dietary and physical activity targets that would be appropriate for each child and parent. Parents were encouraged to meet with their children to set individual goals within the suggested range Increased physical activity encouraged through a pedometer‐based step programme as described above Changes in dietary habits were addressed via a modified version of the Stoplight Diet Set daily dietary goals at the end of each group session, which included limiting the consumption of high‐fat/high‐sugar foods (i.e. "red foods") and increasing fruit and vegetable intake. Encouraged to eat a well‐balanced diet based on the food guide pyramid Parents participated in role‐play activities to practice negotiation of goals with their child. As children were not attending group sessions, an emphasis was placed on teaching parents how to work with their children to set goals together. Parents encouraged to utilise praise, incentives and modelling to encourage participation and goal achievement. Parents provided handouts to guide them in discussing programme material and setting weekly goals with their children. Parents weighed every other group session to monitor their weight status Delivered as described below Behavioural family‐based intervention: 12 sessions of 90 minutes. Parent‐child dyads participated in simultaneous but separate groups. The primary objectives were to build healthier dietary habits, increase moderate intensity physical activity, establish a healthier weight status and build positive self worth 1) In the parent group: the first portion of the meeting involved a review of the progress made in implementing the strategies developed for changing their eating and exercise habits. Difficulties reported by the parents were addressed through group support and discussion. The second segment focused on knowledge and skill training related to nutrition, physical activity and behaviour management strategies At the end of each session, children and parents were brought together to develop goals for the week and specific plans to achieve these goals 2) The child group sessions included 3 segments: a review of progress during the previous week, a physical activity to demonstrate strategies to keep active and preparation of a healthy snack Increased physical activity encouraged through a pedometer‐based step programme. Children and parents encouraged to monitor their physical activity and gradually increase their steps per day. Programme goals based on their baseline level of steps and targeting an increase of at least 3000 steps per day by the end of the programme. Goals set for gradually decreasing sedentary activities (children spend no more than 2 hours per day watching TV or playing video games). If excessive TV viewing was not a concern for a given family, group leaders targeted non‐homework‐based computer time Changes in dietary habits addressed via a modified version of the Stop‐Light programme. Child and parent participants monitored everything they ate using a daily habit log. Goals were individualised to the needs of each family and based on each individual's baseline dietary intake and progress (i.e. goal attainment, weight change) over the course of the programme. Daily dietary goals set each week, including limiting the consumption of high‐fat/high‐sugar "red foods" (with an absolute minimum goal of 2 red foods per day), and increasing fruit and vegetable intake. Children and adults encouraged to eat a well‐balanced diet based on the food guide pyramid Incentivised by providing payment for transportation costs (USD 5 per session) and USD 50 for completing post‐treatment and 6‐month assessment visits Delivered by Family and Consumer Sciences Agents, in collaboration with a post‐doctoral clinical psychologist and graduate students in clinical health psychology who had extensive training and certification in the treatment protocols. The principal investigator of the study conducted periodic direct observation of group sessions to monitor interventionist's performance and assess treatment fidelity. The interventionists also participated in weekly supervision meetings to review each family's progress, discuss group interactions and prepare for the next group session Waiting list control: Families assigned to the waiting list control condition completed the assessment protocol at baseline, and at 4 and 10 months. After the follow‐up period (month 10), families were invited to participate in a 12‐session behavioural‐based intervention. No treatment was delivered until after the final, 6‐month follow‐up assessment |
|
| Outcomes | Outcomes reported in abstract of publication: BMI z score, self esteem, cost | |
| Study details |
Run‐in period: none Study terminated before regular end (for benefit/because of adverse events): no |
|
| Publication details |
Language of publication: English Non‐commercial funding Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "The goals of the study are to (a) assess the feasibility of recruitment in rural settings, (b) develop and evaluate training protocol for group leaders, (c) determine strategies to increase adherence to monitoring and goal setting protocol, (d) evaluate strategies for participant retention, (e) assess the relative cost‐effectiveness of the interventions, (f) assess the acceptability of the intervention to families and Cooperative Extension administrators and personnel, and (g) if successful, estimate the sample size needed for a full scale trial" | |
| Notes | There were 2 comparisons of relevance to this review: the parent‐only vs. parent‐child group and the parent‐only vs. the waiting list control group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: during the initial telephone screen, families were informed as to which 2 evenings the group intervention sessions would be held in their county. Families then indicated which of these evenings they were available to attend. Before the baseline assessment, all families were randomised via computer assignment, based on availability, to 1 of the 2 specific week nights or the waiting list control condition. After randomisation of all families, the interventions (parent‐only or family based) were assigned randomly to the specific week nights |
| Allocation concealment (selection bias) | Unclear risk | Quote from study author: "Randomization was conducted by a research team member who did not participate in assessments. Assignments were written down and put in an envelope by the person making the assignments for each dyad. Envelope was opened with family at end of baseline visit" Comment: unclear whether envelopes were opaque |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Quote from study author: "Participants and treatment personnel were not blind" Comment: families were notified of their group assignment at pre‐treatment assessment |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Quote from study author: "Participants and treatment personnel were not blind" Comment: families were notified of their group assignment at pre‐treatment assessment |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Quote from study author: "Outcome assessors were blinded in that we used personnel to complete outcome assessments that did not serve as treatment personnel in each respective county" |
| Blinding of outcome assessment (detection bias) Objective outcome | Unclear risk | Quote from study author: "Outcome assessors were blinded in that we used personnel to complete outcome assessments that did not serve as treatment personnel in each respective county" |
| Incomplete outcome data (attrition bias) Subjective outcomes | High risk | Comment: no ITT analysis. Numbers completing assessment provided only |
| Incomplete outcome data (attrition bias) Objective outcomes | High risk | Comment: no ITT analysis. Numbers completing assessment provided only |
| Selective reporting (reporting bias) | High risk | Comment: collected other measures that were not reported |
| Other bias | Unclear risk | Comment: not enough information to judge |
Golley 2007.
| Methods |
Parallel randomised controlled trial Randomisation ratio: not reported Superiority design |
|
| Participants |
Inclusion criteria: overweight (according to the International Obesity Task Force definition), and Tanner stage with a carer willing to attend sessions and able to read and understand English Exclusion criteria: BMI z score > 3.5, diagnosed with a syndromal cause of obesity, using medications that influence weight gain or loss, a diagnosis of physical or developmental disability or chronic illness, and a sibling enrolled in the study Diagnostic criteria: overweight (defined as above) |
|
| Interventions |
Number of study centres: 2 Treatment before study: none Titration period: none Detailed description of interventions: Parenting‐skills training: 11 sessions over 24 weeks, 4 weekly 2‐hour group sessions followed by 4 weekly, then 3 monthly 15‐ to 20‐minute individual telephone sessions. Parenting‐skills training was used to facilitate and support parents to undertake family lifestyle change. Positive, Parenting Program (Triple P) based on child development theory and social learning principles and aimed to promote parental competence to manage their child's behaviour. Standard Triple P resource materials were used with programme examples adapted to reflect dietary and activity behaviours. Application of Triple P to eating and activity behaviours was supported by provision of a general healthy lifestyle pamphlet The Triple P Selected Seminar Series consisted of 3 x 2‐hour seminars covering: 1. positive parenting; 2. raising confident and competent‐children, and; 3. raising resilient‐children Learning outcomes ‐ to practitioners (which some is applicable to parents)
Triple P Discussion Groups topics are:
Intervention delivered using standard protocols and a single, trained facilitator to limit site bias and enhance internal study validity. Sessions taken by a dietician Parenting‐skills training with intensive lifestyle education: As above in addition to: 7 intensive lifestyle support group sessions that focused on lifestyle knowledge and skills including the following: family‐focused healthy eating with specific core food serve recommendations, monitoring, label reading, snacks, modifying recipes, being active in a variety of ways, roles and responsibilities around eating, managing appetite, self esteem and teasing While parents attended the lifestyle sessions, children in the group attended structured, supervised activity sessions developed by physical activity experts. The sessions consisted of fun, non‐competitive games designed around aerobic activity and development of fundamental motor skills. Sessions were designed as play rather than exercise and were diversional rather than interventional. The activities required minimal equipment and were deliverable by non‐expert staff and easily replicated at home. Sessions taken by a dietician Waiting list control: Received the same general healthy‐lifestyle pamphlet as the parenting‐alone group. During the 12‐month waiting list period, the waiting list control group was contacted by telephone 3 or 4 times for 5 minutes as a retention strategy. Researcher contact with the waiting list families was minimised to avoid the potential placebo effect of therapist contact |
|
| Outcomes | Outcomes reported in abstract of publication: BMI z score, waist circumference z score | |
| Study details |
Run‐in period: none Study terminated before regular end (for benefit/because of adverse events): no |
|
| Publication details |
Language of publication: English Non‐commercial funding Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "Our goal was to evaluate the relative effectiveness of parenting‐skills training as a key strategy for the treatment of overweight children. The aim of this study was to evaluate the relative effectiveness of parenting‐skills training as a key strategy for the treatment of overweight children" | |
| Notes | There were 3 comparisons of relevance to this review: the parent‐only intensive education group vs. the parent‐only group; the parent‐only + intensive education group vs. the waiting list control; the parent‐only group vs. the waiting list control | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: "Randomization schedules were computer generated using a 3‐block design stratified for gender and site of recruitment..." Comment: researchers involved in recruitment, participant allocation and intervention delivery or data collection were not involved in the randomisation process |
| Allocation concealment (selection bias) | Low risk | Quote from publication: "Individual group allocations were sealed in opaque envelopes, with the next envelope opened on a child's completion of baseline measurements..." |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Comment: carers and participants masked to allocation to treatment assignment but unclear if this also related to blinding throughout the trial |
| Blinding of outcome assessment (detection bias) Objective outcome | Low risk | Quote from publication: "...Data collection was performed by the same trained assessor who was blinded to participant group allocation" Comment: outcome assessment was masked |
| Incomplete outcome data (attrition bias) Objective outcomes | Unclear risk | Quote from publication: "Intention‐to‐treat analysis was performed, with all participants included in the analysis according to original group allocation, and follow‐up was maximized regardless of program attendance" Comment: states ITT but the total number did not match |
| Selective reporting (reporting bias) | High risk | Comment: health‐related quality of life and satisfaction stated as outcomes but not reported |
| Other bias | Unclear risk | Comment: not enough detail to judge |
Golan 2006.
| Methods |
Parallel randomised controlled trial Randomisation ratio: not reported Superiority design |
|
| Participants |
Inclusion criteria: children > 20% overweight (BMI for age and sex > 85th percentile). Parents agreed to attend programme meetings. No current participation of any family member in a weight‐loss programme. No restriction regarding participation in a physical activity programme for children and parents. No diagnosis of psychiatric or major endocrine pathology Exclusion criteria: current participation of any family member in a weight‐loss programme; restriction regarding participation in a physical activity programme for children and parents; diagnosis of psychiatric or major endocrine pathology Diagnostic criteria: BMI for age and sex > 85th percentile |
|
| Interventions |
Number of study centres: 1 Treatment before study: none Titration period: none Description of interventions: Parent‐only group: 16 x 1 hour sessions at the following intervals: weeks 1‐10 (10 sessions); bi‐weekly ‐ weeks 11‐18 (4 sessions); monthly ‐ weeks 19‐26 (2 sessions). Nurturing the child emotionally. Problem solving. The first 3 sessions focused on nutrition education and parental modelling. In the next 2 sessions, the use of an authoritative feeding style was discussed. Sessions 6 and 7 focused on eating and activity behaviour modification, reinforcing means to influence a child's food preferences, as well as employing behaviour modification. Sessions 8 and 9 focused on problem solving while implementing the change in the home. Sessions 10 and 11 dealt with cognitive restructuring and media management. Session 12 focused on coping with resistance. In the remaining 4 sessions, groups discussed their successes and difficulties, as well as recommendations on how to work around constraints imposed on parents in order to promote a healthy lifestyle for all family members Physical activity goals of 4 hours per week, and decrease in sedentary behaviours (to < 3 hours/day) Theoretical basis: parents as the exclusive agent of change. Authoritative feeding style. Nurturing. Parental modelling. Behaviour change. Based on previous work Structured 12‐session programme (unclear if a manual). A clinical dietician, supervised by a family therapist, administered the programme. Training of dietician not reported Parent and children group: Similar in content; however, adapted to fit the children included |
|
| Outcomes | Outcomes reported in abstract of publication: % overweight at end of programme (6 months) and 1‐year follow‐up. Food stimuli in the home (from Family Eating and Activity questionnaire). Parents' weight | |
| Study details |
Run‐in period: none Study terminated before regular end (for benefit/because of adverse events): no |
|
| Publication details |
Language of publication: English Funding not stated Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "The question remains, which is better: parents only or parent and child treatment? The present study extends this knowledge by comparing targeting parents and child versus parents alone, to address the question: Do the children need to be involved at all?..." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: randomisation stratified by age groups: participants divided into age groups: 6‐7 years, 8‐9 years, 10‐11 years, then randomised. No details of randomisation schedule |
| Allocation concealment (selection bias) | Low risk | Quote from publication: "The process was carried out by using two concealed opaque envelopes indicating group 1, namely parents‐only, or group 2, parents and children" |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Unclear risk | Comment: no details provided |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Comment: no details provided |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Quote from publication: "The data were gathered by an MSc [Master of Science] student who was blinded to the treatment allocation" |
| Blinding of outcome assessment (detection bias) Objective outcome | Low risk | Quote from publication: "The data were gathered by an MSc student who was blinded to the treatment allocation" |
| Incomplete outcome data (attrition bias) Subjective outcomes | Low risk | Comment: ITT analysis where the missing values were replaced with baseline values. Reasonable attendance in both arms and numbers and reasons for drop‐outs given |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | Comment: ITT analysis where the missing values were replaced with baseline values. Reasonable attendance in both arms and numbers and reasons for drop‐outs given |
| Selective reporting (reporting bias) | Unclear risk | Comment: all outcomes reported as stated but not enough information to judge |
| Other bias | Unclear risk | Comment: although power calculations given, 12 participants in each arm seems a small number from the power calculation |
Aragona 1975.
| Methods |
Parallel randomised controlled trial Randomisation ratio: superiority Superiority design: 1 : 1 : 1 |
|
| Participants |
Inclusion criteria: overweight girls aged 5‐10 years. Children considered overweight if both their physician and parents recommended that they participate in the weight‐loss programme Exclusion criteria: undergoing psychotherapy, drug therapy or involved in a weight‐reduction programme Diagnostic criteria: not stated |
|
| Interventions |
Number of study centres: assume 1 Treatment before study: none Titration period: none Intervention description: Response‐cost plus reinforcement: 12‐week treatment. Parents given daily weight and calorie graphs, a calorie counter guide and an eating diary. Parents also given a weight reduction programme behavioural contract, instruction on daily exercise and an exercise programme (daily calisthenics that increased in difficulty over a 3‐week period, thereafter 30 minutes per day), nutritional information, instructions in stimulus control techniques, a book 'Living with Children' and information in reinforcement techniques, a daily reinforcement diary. At second baseline visit given a response‐cost contract to return the following week with money for deposit and a weight loss goal of between 1 and 2 pounds per week. Deposits were on a sliding scale of income vs. number of dependents and ranged between USD 12‐30. Money could be redeemed in 12 weekly instalments (25% weekly for attendance, 25% for bringing completed graphs and charts to the meeting, 50% if the child lost the agreed weight). Unearned money divided among the successful parents. Children weighed and then sent to a playroom. After the programme there was an 8‐week no contract follow‐up and following that a post follow‐up check 31 weeks later Response‐cost: Parents given the same as the response‐cost plus reinforcement group except did not receive the book, information on reinforcement techniques or the reinforcement diary Control: Informed would be able to participate at a later date (waiting list control) |
|
| Outcomes | Outcomes reported in abstract of publication: weight change | |
| Study details |
Run‐in period: none Study terminated before regular end (for benefit/because of adverse events): no |
|
| Publication details |
Language of publication: English Non‐commercial funding Publication status: peer‐reviewed publication |
|
| Stated aim for study | Quote from publication: "The present study treated with behavioural techniques overweight children 5 to 10 year of age" | |
| Notes | There were 3 comparisons of relevance to this review: parent‐only + reinforcement vs. parent‐only; parent‐only + reinforcement vs. control; parent‐only vs. control | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from publication: "subjects were randomly assigned to one of three groups" Comment: no other details |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Comment: no details |
| Blinding of outcome assessment (detection bias) Objective outcome | Unclear risk | Comment: no details |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | Comment: numbers and reasons for drop‐outs provided, differential drop‐out between groups (small numbers) |
| Selective reporting (reporting bias) | Unclear risk | Comment: not enough information to judge |
| Other bias | Unclear risk | Comment: not enough information to judge |
Note: where the judgement is 'unclear' and the description is blank, the study did not report that particular outcome.
"‐" denotes not reported.
BMI: body mass index; CBT: cognitive behavioural therapy; ITT: intention‐to‐treat; NOURISH: Nourishing Our Understanding of Role modelling to Improve Support and Health; TV: television.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Berry 2007 | Intervention not parent‐only |
| Biotnott 2009 | Duration of intervention/follow‐up < 6 months |
| Bohlin 2012 | Intervention not parent‐only |
| De Bock 2013 | Intervention not parent‐only |
| Dewes 2014 | Intervention not parent‐only |
| Hendrie 2011 | Intervention not parent‐only |
| Hystad 2013 | Intervention not parent‐only |
| John 2009 | Duration of intervention/follow‐up < 6 months |
| Lawson 2015 | Intervention not parent‐only |
| Le Gross 2006 | Duration of intervention/follow‐up < 6 months |
| NHLBI 2008 | Primary prevention study |
| Parra‐Medina 2015 | Intervention not parent‐only |
| Shelton 2007 | Duration of intervention/follow‐up < 6 months |
| Steele 2014 | Intervention not parent‐only |
| Volkenant 2011 | Duration of intervention/follow‐up < 6 months |
| Warschburger 2013 | Intervention not parent‐only |
Characteristics of studies awaiting assessment [ordered by study ID]
Geronilla 1981.
| Methods | No details |
| Participants | Obese children and adolescents |
| Interventions | No details |
| Outcomes | No details |
| Study identifier | Dissertation abstract: 1982‐72486‐001 |
| Official title | A study of weight control in paediatric obesity using mothers as behavior modifiers |
| Stated purpose of study | No details |
| Notes | We were unable to access the full publication of this study |
Gillick 1975.
| Methods | No details |
| Participants | Mothers and their 6‐ to 12‐year‐old obese children |
| Interventions | No details |
| Outcomes | No details |
| Study identifier | Dissertation abstract: 1977‐13293‐001 |
| Official title | Training parents as therapists in the treatment of juvenile obesity |
| Stated purpose of study | No details |
| Notes | We were unable to access the full publication of this study |
Golan 1998.
| Methods |
Parallel randomised controlled trial Randomisation ratio: not reported Superiority design |
| Participants |
Inclusion criteria: aged 6‐11 years; weight > 20% over expected weight for age, height and gender; no history of psychiatric contact for children; and both parents living at home and parental agreement to meet all requirements of the study (check‐ups, questionnaire, group sessions) Exclusion criteria: the main reasons for exclusion were the children's reluctance to undergo blood sampling and the parents' denial of their children being obese or needing treatment Diagnostic criteria: weight > 20% over expected weight for age, height and gender |
| Interventions |
Number of study centres: 1 Treatment before study: none Titration period: none Description of interventions Parent‐only: 14 x 1‐hour group sessions, conducted by clinical dietician, attended only by parents (delivered as 2 groups of 15 parents). 4 sessions ‐ weekly; 4 sessions ‐ bi‐weekly; 6 sessions ‐ every 6 weeks. Also, 5 x 15 minute individual sessions for whole family, during last 6 group sessions Apply behavioural modifications (implement lifestyle change); practice parenting skills (overlap with nutrition advice as well). All instructions were oriented to the family system. At the sessions, the parents were taught to alter the family sedentary lifestyle, provide a prudent diet (reduction of total and saturated fats, increase of mono‐unsaturated fatty acids), decrease the family's exposure to food stimuli, apply behavioural modifications and practise relevant parenting skills. Other topics discussed were limits of responsibilities, parental modelling, cognitive restructuring and coping with resistance Parent's role was to control the quality and pattern of the food environment, but not restrict the amount of food eaten Training of staff not reported, assumed delivered by a dietician Control intervention: Child prescribed calorie‐controlled diet. Children divided into 2 subgroups. 30 x 1‐hour group sessions, by clinical dietician; 8 sessions ‐ weekly; 22 session ‐ biweekly Children taught techniques how to follow a prudent diet, restrict energy intake, increase exercise, control food stimuli, techniques in self monitoring, practise problem solving and cognitive restructuring, and make use of social support. Individual counselling was offered when a child missed the group session or needed extra support |
| Outcomes | Outcomes reported in abstract of publication: drop‐out; mean reduction in percentage overweight; exposure to food stimuli/changes in eating habits |
| Study identifier |
Run‐in period: none Study terminated before regular end (for benefit/because of adverse events): no |
| Official title |
Language of publication: English Non‐commercial funding Publication status: peer‐reviewed journal |
| Stated purpose of study | Quote from publication: "This study examined the reduction in overweight and changes in eating‐related behaviours in obese children treated with a family‐based approach, in which the parents were the exclusive agents of change. Results were compared to the conventional approach in which children are responsible for their own weight loss" |
| Notes | We contacted authors to establish if the outcome of interest had been measured but have not had a response |
Characteristics of ongoing studies [ordered by study ID]
Ball 2012.
| Trial name or title | Acronym: PAC |
| Methods |
Type of study: efficacy study Allocation: randomised Intervention model: parallel assignment Masking: single blind (participants) Primary purpose: weight loss intervention |
| Participants |
Condition: parents of overweight children Enrolment: estimated 90 Inclusion criteria: families are eligible for this study if children are aged 8‐12 years, children present with an age‐ and sex‐specific BMI ≥ 85th percentile, at least 1 parent agrees to attend weekly PAC sessions for 16 weeks and children and parents are fluent in English (verbal and written) Exclusion criteria: not stated |
| Interventions |
Intervention(s): Parents as Agents of Change (PAC) intervention (includes cognitive behavioural therapy) Comparator(s): psycho‐educational‐based intervention Both intervention arms in the trial are the same in frequency of contact (16 sessions), content (identical information is delivered), mode (group format), duration (60‐90 minutes per session), intervention goals (related to nutrition and physical activity) and the number of group leaders (2 per group). The intervention arms differ in how information is conveyed to parents, and how parents work towards attempting, achieving and maintaining healthy cognitive and behavioural changes |
| Outcomes |
Primary outcome(s): BMI z score Secondary outcome(s): lifestyle behaviours, nutrition and physical activity behaviours; Parental Stress Index (PSI); blood pressure; fasting glucose; fasting insulin; HDL cholesterol; LDL cholesterol; total cholesterol; triglycerides (child only); Family Adaptability and Cohesion Scale‐IV (FACES‐IV). Other outcome(s): as above |
| Starting date |
Study start date: September 2010 Study completion date: January 2014 |
| Contact information | Responsible party/principal investigator: Geoff Ball (gdball@ualberta.ca), University of Alberta, Canada |
| Study identifier | NCT01267097 |
| Official title | Parents as Agents of Change (PAC) in paediatric weight management |
| Stated purpose of study | Quote: "We hypothesize that children with obesity whose parents complete a CBT‐based PAC intervention will achieve greater reductions in adiposity and improvements in cardiometabolic risk factors, lifestyle behaviours, and psychosocial outcomes than children whose parents complete a psycho‐education‐based PAC intervention (PEP)" |
| Notes |
Dalton 2011.
| Trial name or title | Acronym: PLAN |
| Methods |
Type of study: efficacy study Allocation: randomised Intervention model: cluster trial Masking: none Primary purpose: weight loss intervention |
| Participants |
Condition: parents of overweight children Enrolment: estimated 80 Inclusion criteria: BMI ≥ the 85th percentile during the recruitment period, and 1 parent or other primary carer agrees to participate in the study, including individual visits and group sessions as well as telephone follow‐ups. Only one child per family will be included in the study Exclusion criteria: current child or parent/primary carer participation in a weight management programme; presence of a diagnosed psychiatric/psychological disorder in the child or parent/primary carer (e.g. attention deficit hyperactivity disorder, autism, eating disorder); presence of an underlying medical condition affecting weight status (e.g. hypothyroidism, Cushing's syndrome, chronic steroid use); current dietary or physical activity restrictions (e.g. such as in children with diabetes or orthopaedic problems including slipped capital femoral epiphysis); and parents/primary carers do not have telephone accessibility. Parents/primary carers will also have to understand and speak English |
| Interventions |
Intervention(s): parent‐mediated childhood overweight intervention (PLAN) for healthy living and the parent handbook described below. 2 individual visits with clinic provider, 4 clinic‐based group sessions moderated by a trained clinic provider and 4 follow‐up telephone calls from the Project Co‐ordinator or research staff Comparator(s): parents will receive "Families Finding the Balance: A Parent Handbook", a health education handbook adopted by NIH We Can! during the baseline assessment |
| Outcomes |
Primary outcome(s): BMI z score Secondary outcome(s): child's and family's eating and physical activity behaviours and the child's health‐related quality of life Other outcome(s): covariates; healthcare provider perceptions of treatment of child overweight and obesity. Parents and healthcare providers will also complete surveys and focus groups, respectively, on the acceptability and feasibility of this approach including provider perceptions of training |
| Starting date |
Study start date: not reported Study completion date: not reported |
| Contact information | Responsible party/principal investigator: Karen E Schetzina, East Tennessee State University, Johnson City, Tennessee, USA |
| Study identifier | NCT number: NCT01729910 |
| Official title | Parent‐Led Activity and Nutrition (PLAN) for Healthy Living (published protocol) Primary Care Child Obesity Intervention Targeting Parents (trial document) |
| Stated purpose of study | Quote: "1) to establish a primary care based and parent‐mediated childhood overweight intervention program in the primary care setting, 2) to explore the efficacy of this intervention in promoting healthier weight status and health behaviours of children, 3) to examine the acceptability and feasibility of the approach among parents and primary care providers" |
| Notes |
Gerards 2012.
| Trial name or title | Acronym: GO4fit |
| Methods |
Type of study: efficacy study Allocation: randomised Intervention model: parallel assignment Masking: none Primary purpose: weight loss intervention |
| Participants |
Condition: parents of overweight children Enrolment: estimated 84 child‐parent triads Inclusion criteria: parents of children are eligible for participation if their child is considered overweight or obese, based on the BMI, using the international sex‐ and age‐specific cut‐off points proposed by Cole et al. Exclusion criteria: none stated |
| Interventions |
Intervention(s): Lifestyle Triple P intervention for parents with active skills training methods based on self regulation principles, to provide parents with new knowledge and skills. 14‐week intervention, 8 weekly 90‐minute parental group sessions, followed by 2 weekly 15‐30 minute telephone sessions, 1 further 90‐minute group session, 2 weekly 15‐ to 30‐minute telephone sessions, and a final 90‐minute group session Comparator(s): receive 2 brochures (1 on healthy nutrition and physical activity, and 1 on positive parenting), web‐based tailored advice on setting a good example to their child, and suggestions for exercises to increase active play at home |
| Outcomes |
Primary outcome(s): BMI z score, waist circumference, fat mass Secondary outcome(s): children's dietary behaviour and physical activity level, parenting practices, parental feeding style, parenting styles, parental self efficacy, and body composition of family members (parents and siblings) Other outcome(s): as above |
| Starting date |
Study start date: December 2010 Study completion date: December 2012 |
| Contact information | Responsible party/principal investigator: Sanne Gerards (sanne.gerards@maastrichtuniversity.nl), Maastricht University, The Netherlands |
| Study identifier | The Netherlands National Trial Register NTR2555 |
| Official title | Lifestyle Triple P: a parenting intervention for childhood obesity (published protocol) Effectiveness of Lifestyle Triple P: an intervention aimed at the prevention of excessive weight gain in 4‐ till 8‐year‐old overweight children. ‐ GO4fit (trial document) |
| Stated purpose of study | Quote: "The aim of the current randomised controlled trial is to assess the effectiveness of the Lifestyle Triple P intervention when applied to Dutch parents of overweight and obese children aged 4–8 years" |
| Notes |
Janicke 2011.
| Trial name or title | Acronym: E‐FLIP for Kids |
| Methods |
Type of study: efficacy study Allocation: randomised Intervention model: parallel assignment Masking: not reported Primary purpose: treatment |
| Participants |
Condition: overweight and obese Enrolment: estimated 240 Inclusion criteria: aged 8‐12 years, BMI ≥ 85th percentile for age and gender, living within the same dwelling in a rural county. Participating parents or legal guardians must be age ≤ 75 years Exclusion criteria: participating parent has a dietary or exercise restriction, or a medical condition that contraindicates mild energy restriction or moderate physical activity. Children or participating parents on antipsychotic agents, systemic corticosteroids, antibiotics for HIV or tuberculosis, chemotherapeutic drugs or who are currently using prescription weight‐loss drugs, child has a resting blood pressure ≥ 140/90 mmHg, not engaged in another weight control programme, exhibit conditions or behaviours that are likely to affect their participation in the trial, such as being unwilling or unable to give informed consent, parent(s) or legal guardian(s) unable to read English at approximately the 5th grade level, unwilling to accept random assignment, unable to travel to the extension office for intervention sessions, or likely to move out of the county within the next 24 months |
| Interventions |
Interventions: 1. General intervention + parent‐only intervention: weekly group sessions for the first 8 weeks, then 4 biweekly sessions over the next 8 weeks for a total of 12 sessions across 16 weeks. Contact will then fade to 1 group session per month for months 5‐12, with the exception of month 9, during which participants will attend 2 group sessions. Each session will last 90 minutes. Focus on diet, physical activity and behavioural components 2. General intervention + family‐based behavioural intervention: as above but involves children and parents who will meet in simultaneous, but separate, parent‐child groups at each meeting Comparator(s): 21 group meetings on the same schedule as the intervention arms. Each session will last 90 minutes. The families in the Health Education group will not receive training in behavioural self regulation strategies, such as goal setting and self monitoring. There will be no instruction on behavioural parenting strategies and parent |
| Outcomes |
Primary outcome(s): BMI z score Secondary outcome(s): child body fat, waist circumference, height, weight, dietary intake, physical activity, blood lipids, blood glucose, parental measures, health‐related quality of life, parenting skills, costs and cost effectiveness |
| Starting date |
Study start date: July 2009 Study completion date: December 2014 |
| Contact information | Responsible party/principal investigator: David Janicke (djanicke@phhp.ufl.edu) |
| Study identifier | NCT number: NCT01820338 |
| Official title | The Extension Family Lifestyle Intervention Project (E‐FLIP for Kids) (published protocol and trial document) |
| Stated purpose of study | Quote: "assessing the effectiveness of two behavioral weight management interventions in an important and at‐risk population, overweight and obese children and their parents in rural counties..." |
| Notes |
NCT01197443.
| Trial name or title | Acronym: PAAC |
| Methods |
Type of study: efficacy and cost effectiveness study Allocation: randomised Intervention model: parallel assignment Masking: none Primary purpose: weight loss intervention |
| Participants |
Condition: parents of overweight children Enrolment: estimated 150 Inclusion criteria: overweight child > 95th percentile for age and gender; an overweight (BMI > 25) parent willing to participate and attend all treatment meetings; eligible parent who can read at a minimum of an 8th grade level; family willing to commit to 5 months of treatment attendance and follow‐up for 18 months post‐treatment Exclusion criteria: major child psychiatric disorder diagnoses; child diagnoses of a serious current physical disease (such as diabetes) for which physician supervision of diet and exercise prescription are needed (self report); family with restrictions on types of food, such as food allergies, religious or ethnic practices that limit the foods available in the home; child with physical difficulties that limit the ability to exercise; child with an active eating disorder (based on Eating Disorder Examination interview); families where children or parents are involved in swimming or weight training more than 5 hours per week; major parent psychiatric disorder |
| Interventions |
Intervention(s): behavioural parent‐only intervention Comparator(s): behavioural parent‐child intervention The treatment length is set for 12 weekly meetings and bi‐monthly meetings during months 4 and 5. Each group session will be 60 minutes including weigh‐ins |
| Outcomes |
Primary outcome(s): weight; BMI for age percentile; BMI z score Secondary outcome(s): cost effectiveness, dietary quality, exercise behaviour, quality of life, psychosocial measures, parenting adherence, parenting style, parent weight loss, compliance and changes in household food environment |
| Starting date |
Study start date: November 2010 Study completion date: July 2015 |
| Contact information | Responsible party/principal investigator: Kerri Boutelle (kboutelle@ucsd.edu), University of California, San Diego, USA |
| Study identifier | NCT number: NCT01197443 |
| Official title | Parents as the Agent of Change for Childhood Obesity (PAAC) |
| Stated purpose of study | Quote: "To evaluate the efficacy of parent only treatment versus parent + child treatment in the body weight of the target child. To evaluate the cost effectiveness compared to current gold standard treatment of parent and child dual education" |
| Notes |
NCT01546727.
| Trial name or title | Acronym: Behavioral Treatment for Obese Preschoolers (LAUNCH) |
| Methods |
Type of study: efficacy Allocation: randomised Intervention model: factorial assignment Masking: double blind Primary purpose: treatment |
| Participants |
Condition: obesity Enrolment: estimated 168 Inclusion criteria: children aged 2 years 0 months to 5 years 11 months; BMI percentile ≥ 95th percentile for age and gender, but no more than 100% above the median BMI for age and gender; English‐speaking; Live within 50 miles of Cincinnati Children's Hospital Medical Center (CCHMC); medical clearance from the child's paediatrician to participate Exclusion criteria: medical conditions known to promote obesity (e.g. Prader‐Willi syndrome, Cushing's syndrome); already involved with another weight control programme; taking weight‐affecting medications (e.g. steroids); a disability or illness that would preclude them from engaging in at least moderate intensity physical activity; developmental disability |
| Interventions |
Intervention(s): 1. Behavioural family intervention. 3 months of weekly treatment delivered via alternating group‐based clinic visits and individual home visits followed by 3 months of every other week treatment alternating between clinic and home 2. Behavioural: motivational interviewing ‐ 4 in‐person visits spaced at first visit, month 3 and month 5. Weekly telephone calls during the first 3 months and every other week during months 4‐6 Comparator(s): standard care |
| Outcomes |
Primary outcomes: BMI z score change Secondary outcome(s): BMI z score; caloric intake; physical activity; home environment; parent caloric intake; parent physical activity; parent and child eating behaviours; health‐related quality of life |
| Starting date |
Study start date: March 2012 Study completion date: November 2016 |
| Contact information | Responsible party/principal investigator: Lori J Stark, Children's Hospital Medical Center, Cincinnati, USA |
| Study identifier | NCT number: NCT01546727 |
| Official title | Clinic and Home Family Based Behavioral Treatment for Obese Preschoolers |
| Stated purpose of study | Quote: "to test a clinic and home family behavioral intervention (LAUNCH) against 1) motivational interviewing (attention control; MI) and 2) standard of care (true standard of care control" |
| Notes |
NCT01552642.
| Trial name or title | Acronym: none |
| Methods |
Type of study: efficacy Allocation: randomised Intervention model: parallel Masking: open label Primary purpose: treatment |
| Participants |
Condition: obesity, overweight Enrolment: estimated 156 Inclusion criteria: 1 parent of a 3‐ to 6‐year old child with a BMI Ͱ5 85th percentile, Internet access, English speaking Exclusion criteria: children with a developmental disorder, children with a chronic underlying disease that may contribute to obesity, children taking medication that may interfere with a healthy weight |
| Interventions |
Intervention: parenting behavioural intervention: 6 weekly face‐to‐face group (10‐12 parents) meetings and access to a website. Contents include authoritative parenting, using the food plan "Go, Slow, and Whoa", increasing physical activity and behaviour change strategies. Parenting skills will be discussed at every session. Website has information and links about nutrition and physical activity, an interactive group session and an ask the expert facility Comparator: no intervention |
| Outcomes |
Primary outcome: feasibility Secondary outcomes: BMI z scores, healthy behaviour changes, parenting skills Other outcome(s): ‐ |
| Starting date |
Study start date: February 2013 Study completion date: August 2015 |
| Contact information | Responsible party/principal investigator: Ellen R Wald, University of Wisconsin, Madison, USA |
| Study identifier | NCT number: NCT01552642 |
| Official title | An Interactive Web‐based Intervention to Achieve Healthy Weight in Young Children |
| Stated purpose of study | Quote: "to develop and implement an effective intervention program designed to prevent and treat obesity in young children" |
| Notes |
NCT01792531.
| Trial name or title | Acronym: More and Less study (M+L) |
| Methods |
Type of study: efficacy Allocation: randomised Intervention model: parallel Masking: open label Primary purpose: treatment |
| Participants |
Condition: obesity Enrolment: estimated 180 Inclusion criteria: age 4‐6 years old; obesity as defined by international cut‐offs Exclusion criteria: weight affecting diseases |
| Interventions |
Intervention(s): parent training group with 2 subgroups, 12‐week only vs. bolster sessions at 8‐week intervals over 1 year Focus on how to use positive parenting practices instead of ineffective practices. 12 sessions (1.5 hours per week), introduction to effective parenting practices, discussion and practice using role play and home practice assignments. Tailored to focus on changes in the home environment, mostly related to child food habits and physical activity Comparator(s): standard treatment with focus on lifestyle, provided by local paediatricians in outpatient paediatric departments and will be based on lifestyle modifications, as recommended in the action plan for Stockholm County |
| Outcomes |
Primary outcome(s): BMI change at 1 year Secondary outcome(s): changes in: parenting practices; child's dietary intake and behaviour; child's physical activity; family functioning; child's metabolic health; parental functioning; waist circumference; child's functioning, socioeconomic status Other outcome(s): ‐ |
| Starting date |
Study start date: January 2013 Study completion date: December 2017 |
| Contact information | Responsible party/principal investigator: Paulina Nowicka, Karolinska Institutet, Sweden |
| Study identifier | NCT number: NCT01792531 |
| Official title | The More & Less Study: A Trial Testing Different Treatment Approaches to Obesity in Preschoolers (M&L) |
| Stated purpose of study | Quote: "to evaluate the effectiveness of early treatment of childhood obesity" |
| Notes |
NCT02373670.
| Trial name or title | Acronym: none |
| Methods |
Type of study: interventional Allocation: randomised Intervention model: parallel Masking: open label Primary purpose: treatment |
| Participants |
Condition: obesity Enrolment: estimated 60 Inclusion criteria: aged 2‐5 years, BMI z score > 2 Exclusion criteria: medical conditions or receiving medications affecting weight |
| Interventions |
Intervention: parent mentors using positive deviance findings to promote healthy behaviours. Using locally derived positive deviance findings to inform a behavioural intervention Comparator: education, community health workers providing health education to promote healthy behaviour. Using standardised healthy behaviour education (EatPlayGrow curriculum from NHLBI) |
| Outcomes |
Primary outcome: BMI z score Secondary outcome(s): not reported Other outcome(s): not reported |
| Starting date |
Study start date: January 2015 Study completion date: June 2015 |
| Contact information | Responsible party/principal investigator: The University of Texas Health Science Center at San Antonio, USA |
| Study identifier | NCT number: NCT02373670 |
| Official title | Parent Mentors Using Positive Deviance in Childhood Obesity |
| Stated purpose of study | Quote: "A feasibility study randomising participants (parents of children age 2‐5 years old) to receive either education or a parent mentor with the aim of improving health behaviours and improving their body mass index z‐score" |
| Notes |
Önnerfält 2012.
| Trial name or title | Acronym: LOOPS |
| Methods |
Type of study:interventional Allocation: randomised Intervention model: parallel Masking: open label Primary purpose: treatment |
| Participants |
Condition: overweight, obese Enrolment: estimated 240 (160 overweight, 80 obese) Inclusion criteria: 4‐ to 6‐year old children with overweight and obesity Exclusion criteria: do not understand written and spoken Swedish well enough to participate in group activities |
| Interventions |
Intervention(s): All start with a 2‐hour lecture with general facts about overweight in children (GFO), performed by health professionals. Also access to a website, Healthy Children (HC), with general information about diet and exercise recommendations Obese children randomised to either:
in the parents'–everyday life will induce changes that will gradually lead to a normalisation of their children's weight. Groups meet for 13 x 2‐hour sessions over 12 months Overweight children randomised to 1 of 3 groups
Parents are invited to attend group meetings with the general purpose of supporting the parents to accomplish preferred lifestyle changes, both in the short and long term Comparator(s): as above |
| Outcomes |
Primary outcome(s): change in BMI z score Secondary outcome(s): dietary and exercise patterns, waist circumference, insulin resistance, dietary hormones, fecal micro‐flora Other outcome(s): parent change in BMI, perception of their own health, parent stress, child feeding and exercise habits |
| Starting date |
Study start date: August 2008 Study completion date: November 2015 |
| Contact information | Responsible party/principal investigator: Kristina Thorngren‐Jerneck (Kristina.Thorngren‐Jerneck@med.lu.se), Lund University Children's hospital, Sweden |
| Study identifier | NCT number: NCT00916318 |
| Official title | Overweight and Obesity in Preschool Children, Prevalence and Prevention ‐ Family Based Health Interventions for Child Health (trial document) LOOPS ‐ Lund Overweight and Obesity Preschool Study (published protocol) |
| Stated purpose of study | Quote: "to evaluate if a family‐based intervention, targeting parents of preschool children with overweight and obesity, has a long‐term positive effect on weight development of the children" |
| Notes |
BMI: body mass index; HDL: high‐density lipoprotein; LDL: low‐density lipoprotein.
Differences between protocol and review
Given the rapid growth in the treatment of child and adolescent obesity, the original review was split into six separate reviews, with a specific intervention and age focus.
Diet, physical activity and behavioural interventions for the treatment of overweight or obesity in adolescents aged 12 to 17 years.
Diet, physical activity and behavioural interventions for the treatment of overweight or obesity in children aged 5 to 11 years.
Diet, physical activity and behavioural interventions for the treatment of overweight or obesity in infants aged 0 to 4 years.
Drug interventions for the treatment of obesity in children and adolescents.
Parent‐only interventions for childhood overweight or obesity.
Surgery for the treatment of obesity in children and adolescents.
For lifestyle interventions, we included only randomised controlled trials that were specifically designed to treat obesity in children and observed participants for a minimum of six months. The rationale for introducing this criterion arose from the belief that many interventions appear to be effective in the short term (up to three months), but not in the long term (Glenny 1997). It seemed to be more important to evaluate the longer‐term effects of treatments, as this would provide a more valuable indication of effectiveness, given the chronic nature of obesity.
Contributions of authors
Emma Loveman (EL): acquiring trial reports, trial selection, data extraction, data analysis, data interpretation, review draft and future review updates.
Lena Al‐Khudairy (LAK): acquiring trial reports, trial selection and data extraction.
Rebecca E Johnson (RJ): acquiring trial reports, trial selection and data extraction.
Wendy Robertson (WR): acquiring trial reports, trial selection, review draft and future review updates.
Jill L Colquitt (JC): acquiring trial reports, trial selection and data extraction.
Emma L Mead (EM): acquiring trial reports and trial selection.
Louisa J Ells (LE): acquiring trial reports and trial selection.
Maria‐Inti Metzendorf (MIM): search strategy development and review draft.
Karen Rees (KR): project management, trial selection, resolving discrepancies, data interpretation, review draft and future review updates.
Sources of support
Internal sources
Division of Health Sciences, Warwick Medical School, University of Warwick, UK.
Health and Social Care Institute, Teesside University, UK.
Effective Evidence LLP, UK.
External sources
NIHR Cochrane Programme Grant, UK.
Karen Rees, Lena Al‐Khudairy, Rebecca Johnson and Wendy Robertson are also supported by the National Institute for Health Research Collaboration for Leadership in Applied Health Research and Care West Midlands at University Hospitals Birmingham NHS Foundation Trust, UK.
Declarations of interest
EL: none known.
LAK: none known.
RJ: none known.
WR: none known.
JC: none known.
EM: none known.
LE: none known.
MIM: none known.
KR: none known.
New
References
References to studies included in this review
Aragona 1975 {published data only}
- Aragona J, Cassady J, Drabman RS. Treating overweight children through parental training and contingency contracting. Journal of Applied Behavior Analysis 1975;8(3):269‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boutelle 2011 {published data only}
- Boutelle KN, Cafri G, Crow SJ. Parent‐only treatment for childhood obesity: a randomized controlled trial. Obesity 2011;19:574‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Collins 2011 {published data only}
- Burrows T, Janet WM, Collins CE. Long‐term changes in food consumption trends in overweight children in the HIKCUPS intervention. Journal of Pediatric Gastroenterology and Nutrition 2011;53(5):543‐7. [DOI] [PubMed] [Google Scholar]
- Burrows T, Warren JM, Baur LA, Collins CE. Impact of a child obesity intervention on dietary intake and behaviors. International Journal of Obesity 2008;32(10):1481‐8. [DOI] [PubMed] [Google Scholar]
- Burrows T, Warren JM, Collins CE. The impact of a child obesity treatment intervention on parent child‐feeding practices. International Journal of Pediatric Obesity 2010;5(1):43‐50. [DOI] [PubMed] [Google Scholar]
- Cliff DP, Okely AD, Morgan PJ, Steele JR, Jones RA, Colyvas K, et al. Movement skills and physical activity in obese children: randomized controlled trial. Medicine and Science in Sports and Exercise 2011;43(1):90‐100. [DOI] [PubMed] [Google Scholar]
- Collins CE, Morgan PJ, Okely AD, Burrows TL, Cliff DP, Jones RA, et al. HIKCUPS (Hunter Illawarra Kids Challenge Using Parent Support) reduces BMI z‐score up to 2 years: results of a multi‐site randomized trial for overweight children. Obesity Reviews 2010;11:280. [Google Scholar]
- Collins CE, Okely AD, Morgan PJ, Jones RA, Burrows TL, Cliff DP, et al. Parent diet modification, child activity, or both in obese children: an RCT. Pediatrics 2011;127:619‐27. [DOI] [PubMed] [Google Scholar]
- Jones RA, Okely AD, Collins CE, Morgan PJ, Steele JR, Warren JM, et al. The HIKCUPS trial: a multi‐site randomized controlled trial of a combined physical activity skill‐development and dietary modification program in overweight and obese children. BMC Public Health. 2007;7:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okely AD, Collins CE, Morgan PJ, Jones RA, Warren JM, Cliff DP, et al. Multi‐site randomized controlled trial of a child‐centered physical activity program, a parent‐centered dietary‐modification program, or both in overweight children: the HIKCUPS study. Journal of Pediatrics 2010;157(3):388‐94. [DOI] [PubMed] [Google Scholar]
Esfarjani 2013 {published data only}
- Esfarjani F, Khalafi M, Mohammadi F, Mansour A, Roustaee R, Zamani‐Nour N, et al. Family‐based intervention for childhood obesity: an experience among Tehranian children. Annals of Nutrition and Metabolism 2013;63:844. [PMC free article] [PubMed] [Google Scholar]
- Esfarjani F, Khalafi M, Mohammadi F, Mansour A, Roustaee R, Zamani‐Nour N, et al. Family‐based intervention for controlling childhood obesity: an experience among Iranian children. International Journal of Preventive Medicine 2013;4(3):358‐65. [PMC free article] [PubMed] [Google Scholar]
Estabrooks 2009 {published data only}
- Estabrooks PA, Shoup JA, Gattshall M, Dandamudi P, Shetterly S, Xu S. Automated telephone counseling for parents of overweight children: a randomized controlled trial. American Journal Preventive Medicine 2009;26(1):35‐42. [DOI] [PubMed] [Google Scholar]
Golan 2006 {published data only}
- Golan M. Parents as agents of change in childhood obesity ‐ from research to practice. International Journal of Pediatric Obesity 2006;1:66‐76. [DOI] [PubMed] [Google Scholar]
- Golan M, Kaufman V, Shahar DR. Childhood obesity treatment: targeting parents exclusively v. parents and children. British Journal of Nutrition 2006;95:1008‐15. [DOI] [PubMed] [Google Scholar]
Golley 2007 {published data only}
- Golley RK, Magarey AM, Baur LA, Steinbeck KS, Daniels LA. Twelve‐month effectiveness of a parent‐led, family‐focused weight‐management program for prepubertal children: a randomized, controlled trial. Pediatrics 2007;119(3):517‐25. [DOI] [PubMed] [Google Scholar]
- Golley RK, Magarey AM, Daniels LA. Children's food and activity patterns following a six‐month child weight management program. International Journal of Pediatric Obesity 2011;6:409‐14. [DOI] [PubMed] [Google Scholar]
Janicke 2008 {published data only}
- Janicke DM, Sallinen BJ, Perri MG, Lutes LD, Huerta M, Silverstein JH, et al. Comparison of parent‐only versus family‐based interventions for overweight children in underserved rural settings: outcomes from Project STORY. Archives of Pediatrics and Adolescent Medicine 2008;162:1119‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janicke DM, Sallinen BJ, Perri MG, Lutes LD, Silverstein JH, Brumback B. Comparison of program costs for parent‐only and family‐based interventions for pediatric obesity in medically underserved rural settings. Journal of Rural Health 2009;25:326‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janicke DM, Sallinen BJ, Perri MG, Lutes LD, Silverstein JH, Huerta MG, et al. Sensible treatment of obesity in rural youth (STORY): design and methods. Contemporary Clinical Trials 2008;29(2):270‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker K. Mechanisms of self‐esteem change in overweight children participating in a family‐based weight management program. Dissertation. University of Florida. 2007.
Jansen 2011 {published data only}
- Jansen E, Mulkens S, Jansen A. Tackling childhood overweight: treating parents exclusively is effective. International Journal of Obesity (Lond) 2011;35(4):501‐9. [DOI] [PubMed] [Google Scholar]
Magarey 2011 {published data only}
- Magarey AM, Perry RA, Baur LA, Steinbeck KS, Sawyer M, Hills AP, et al. A parent‐led family‐focused treatment program for overweight children aged 5 to 9 years: the PEACH RCT. Pediatrics 2011;127(2):214‐22. [DOI] [PubMed] [Google Scholar]
Mazzeo 2014 {published data only}
- Bean MK, Wilson DB, Thornton LM, Kelly N, Mazzeo SE. Dietary intake in a randomized‐controlled pilot of NOURISH: a parent intervention for overweight children. Preventive Medicine 2012;55(3):224‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzeo SE, Kelly NR, Stern M, Gow RW, Cotter EW, Thornton LM, et al. Parent skills training to enhance weight loss in overweight children: evaluation of NOURISH. Eating Behavior 2014;15(2):225‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzeo SE, Kelly NR, Stern M, Gow RW, Serdar K, Evans RK, et al. Nourishing Our Understanding of Role Modeling to Improve Support and Health (NOURISH): design and methods. Contemporary Clinical Trials 2012;33(3):515‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moens 2012 {published data only}
- Moens E, Braet C. Training parents of overweight children in parenting skills: a 12‐month evaluation. Behaviour. 2012;40(1):1‐18. [DOI] [PubMed] [Google Scholar]
Munsch 2008 {published data only}
- Munsch S, Roth B, Michael T, Meyer AH, Biedert E, Roth S, et al. Randomized controlled comparison of two cognitive behavioral therapies for obese children: mother versus mother‐child cognitive behavioral therapy. Psychotherapy and Psychosomatics 2008;77:235‐46. [DOI] [PubMed] [Google Scholar]
- Roth B, Munsch S, Meyer AH. Long‐term evaluation of a psychological training for obese children and their parents (TAKE) [Langzeitevaluation eines psychologischen Trainingsfür adipöse Kinder und ihre Eltern (TAKE)]. Praxis der Kinderpsychologie und Kinderpsychiatrie 2011;60:304‐21. [DOI] [PubMed] [Google Scholar]
Raynor 2012a {published data only}
- Raynor HA, Osterholt KM, Hart CN, Jelalian E, Vivier P, Wing RR. Efficacy of US pediatric obesity primary care guidelines: two randomized trials. Pediatric Obesity 2012;7(1):28‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Raynor 2012b {published data only}
- Raynor HA, Osterholt KM, Hart CN, Jelalian E, Vivier P, Wing RR. Efficacy of US pediatric obesity primary care guidelines: two randomized trials. Pediatric Obesity 2012;7(1):28‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Resnick 2009 {published data only}
- Resnick EA, Bishop M, O'Connell A, Hugo B, Isern G, Timm A, et al. The CHEER study to reduce BMI in Elementary School students: a school‐based, parent‐directed study in Framingham, Massachusetts. Journal of School Nursing 2009;25(5):361‐72. [DOI] [PubMed] [Google Scholar]
Resnicow 2015 {published data only}
- Resnicow K, McMaster F, Bocian A, Harris D, Zhou Y, Snetselaar L, et al. Motivational nterviewing and dietary counseling for obesity in primary care: an RCT. Pediatrics 2015;135:649‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Small 2013 {published data only}
- Small L, Bonds‐McClain D. A primary care‐based randomized treatment trial with overweight/obese children. Communicating Nursing Research 2013;46:291. [Google Scholar]
- Small L, Bonds‐McClain D, Melnyk B, Vaughan L, Gannon AM. The preliminary effects of a primary care‐based randomized treatment trial with overweight and obese young children and their parents. Journal of Pediatric Health Care 2014;28(3):198‐207. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Grieken 2013 {published data only}
- Veldhuis L, Struijk MK, Kroeze W, Oenema A, Renders CM, Bulk‐Bunschoten AM, et al. "Be active, eat right", evaluation of an overweight prevention protocol among 5‐year‐old children: design of a cluster randomised controlled trial. BMC Public Health 2009;9:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieken A, Renders CM, Veldhuis L, Looman CW, Hirasing RA, Raat H. Promotion of a healthy lifestyle among 5‐year‐old overweight children: health behavior outcomes of the 'Be active, eat right' study. BMC Public Health 2014;14:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieken A, Veldhuis L, Renders CM, Borsboom GJ, Wouden JC, Hirasing RA, et al. Population‐based childhood overweight prevention: outcomes of the 'Be Active, Eat Right' study. PLoS One 2013;8:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
West 2010 {published data only}
- West F, Sanders MR, Cleghorn GJ, Davies PS. Randomised clinical trial of a family‐based lifestyle intervention for childhood obesity involving parents as the exclusive agents of change. Behaviour Research and Therapy 2010;48(12):1170‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Berry 2007 {published data only}
- Berry D, Savoye M, Melkus G, Grey M. An intervention for multiethnic obese parents and overweight children. Applied Nursing Research 2007;20(2):63‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Biotnott 2009 {published data only}
- Boitnott AD. Childhood obesity: development of a parent‐focused intervention. Dissertation Abstracts International: Section B: The Sciences and Engineering 2009;7(3):1588. [Google Scholar]
Bohlin 2012 {published data only}
- Bohlin A, Klaesson S, Kowalski J. Can telephone consultations substitute visits in treatment of childhood obesity? Results from a randomized trial. Obesity Facts 2012;5:237. [Google Scholar]
De Bock 2013 {published data only}
- Bock F, Fischer JE, Hoffmann K, Renz‐Polster H. A participatory parent‐focused intervention promoting physical activity in preschools: design of a cluster‐randomized trial. BMC Public Health 2010;10:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock F, Genser B, Raat H, Fischer JE, Renz‐Polster H. A participatory physical activity intervention in preschools: a cluster randomized controlled trial. American Journal of Preventive Medicine 2013;45(1):64‐74. [DOI] [PubMed] [Google Scholar]
Dewes 2014 {published data only}
- Dewes O, Sluyter J, Scragg R, Jiang Y, Percival T. Fanau FAB: parent‐focused weight management programme for Pacific children. Obesity Reviews 2014;15:212. [Google Scholar]
Hendrie 2011 {published data only}
- Hendrie GA, Golley RK. A parent led intervention targeting purchase of low fat dairy foods reduces saturated fat intake without a change in energy, BMI or negative food consequences. Obesity Research and Clinical Practice 2011;5:S28. [Google Scholar]
Hystad 2013 {published data only}
- Hystad HT, Steinsbekk S, Ødegard R, Wichstrøm L, Gudbrandsen OA. A randomised study on the effectiveness of therapist‐led v. self‐help parental intervention for treating childhood obesity. British Journal of Nutrition 2013;110:1143‐50. [DOI] [PubMed] [Google Scholar]
John 2009 {unpublished data only}
- John R. Effects of Parent‐Focused Media Interventions on Body Mass Index, Waist Size, Self‐Perception, Family Eating Habits, and Family Activity Habits in Overweight Hispanic Children [Thesis]. New York: Teachers College, Columbia University, 2009. [Google Scholar]
Lawson 2015 {published data only}
- Lawson Health Research. FOR HEALTH: a family‐oriented healthy eating, activity and lifestyle intervention for overweight preschool children, 2015. ClinicalTrials.gov/show/NCT01698606 (accessed 16 December 2015).
Le Gross 2006 {published data only}
- The "Kids on Track" program, 2006. www.anzctr.org.au/ACTRN12606000382572.aspx (accessed 15 December 2015).
NHLBI 2008 {published data only}
- National Heart Lung Blood Institute. Family‐based nutrition intervention for Latino children, 2008. ClinicalTrials.gov/show/NCT00224887 (accessed 15 December 2015).
Parra‐Medina 2015 {published data only}
- University of Texas Health Science Center. Health4Kids intervention trial for Hispanic families, 2015. ClinicalTrials.gov/show/NCT02343367 (accessed 15 December 2015).
Shelton 2007 {published data only}
- Shelton D, LeGros K, Norton L, Stanton‐Cook S, Morgan J, Masterman P. Randomised controlled trial: a parent‐based group education programme for overweight children. Journal of Paediatrics and Child Health 2007;43:799‐805. [DOI] [PubMed] [Google Scholar]
Steele 2014 {published data only}
- Steele RG, Jensen CD, Gayes LA, Liebold HC. Medium is the message: moderate parental control of feeding correlates with improved weight outcome in a pediatric obesity intervention. Journal of Pediatric Psychology 2014;39(7):708‐17. [DOI] [PubMed] [Google Scholar]
Volkenant 2011 {published data only}
- Volkenant KR, Quinlan NP, Ubinger M, Rukstalis M, Cochran WJ. Early childhood obesity intervention in primary care: parent stress levels and child mental health symptoms. Obesity 2011;19:S108. [Google Scholar]
Warschburger 2013 {published data only}
- Warschburger P, Kroller K, Unverzagt S, Haerting J. What is the parents' part in long‐term weight management of their obese child? Results from the EPOC study. Obesity Facts 2013;6:230. [Google Scholar]
- Warschburger P, Kroller K, Unverzagt S, Haerting J, Egmond‐Fröhlich A. Empowering parents of obese children (EPOC): a randomized‐controlled trial on additional long‐term weight effects of a parent training. Submitted data. [DOI] [PubMed]
- Warschburger P, Kuhne D, Kroller K. Do parents influence the long term development of obese children's weight? First results of the EPOC‐study [in German]. Padiatrische Praxis 2012;79(2):185‐92. [Google Scholar]
References to studies awaiting assessment
Geronilla 1981 {published and unpublished data}
- Geronilla LS. A study of weight control in pediatric obesity using mothers as behavior modifiers. Dissertation Abstracts International 1981;42(5‐B):2027. [Google Scholar]
Gillick 1975 {published and unpublished data}
- Gillick SL. Training parents as therapists in the treatment of juvenile obesity. Dissertation Abstracts International 1975;35(10‐B):5111‐2. [Google Scholar]
Golan 1998 {published data only}
- Golan M, Crow S. Targeting parents exclusively in the treatment of childhood obesity: long‐term results. Obesity Research 2004;12:357‐61. [DOI] [PubMed] [Google Scholar]
- Golan M, Fainaru M, Weizman A. Role of behaviour modification in the treatment of childhood obesity with the parents as the exclusive agents of change. International journal of obesity and related metabolic disorders 1998;22:1217‐24. [DOI] [PubMed] [Google Scholar]
- Golan M, Weizman A, Apter A, Fainaru M. Parents as the exclusive agents of change in the treatment of childhood obesity. American Journal of Clinical Nutrition 1998;67(6):1130‐5. [DOI] [PubMed] [Google Scholar]
- Golan M, Weizman A, Fainaru M. Impact of treatment for childhood obesity on parental risk factors for cardiovascular disease. Preventive Medicine 1999;29(6):519‐26. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Ball 2012 {published data only}
- Ball GD, Ambler KA, Keaschuk RA, Rosychuk RJ, Holt NL, Spence JC, et al. Parents as agents of change (PAC) in pediatric weight management: the protocol for the PAC randomized clinical trial. BMC Pediatrics 2012;12:114. [DOI: 10.1186/1471-2431-12-114.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dalton 2011 {published data only}
- Dalton WT, Schetzina KE, Holt N, Fulton‐Robinson H, Ho AL, Tudiver F, et al. Parent‐Led Activity and Nutrition (PLAN) for healthy living: design and methods. Contemporary Clinical Trials 2011;32(6):882‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gerards 2012 {published data only}
- Gerards SM, Dagnelie PC, Jansen MW, Goot LO, Vries NK, Sanders MR, et al. Lifestyle Triple P: a parenting intervention for childhood obesity. BMC Public Health 2012;12:267. [DOI: 10.1186/1471-2458-12-267] [DOI] [PMC free article] [PubMed] [Google Scholar]
Janicke 2011 {published data only}
- Janicke DM, Lim CS, Perri MG, Bobroff LB, Mathews AE, Brumback BA, et al. The Extension Family Lifestyle Intervention Project (E‐FLIP for kids): design and methods. Contemporary Clinical Trials 2011;32(1):50‐8. [DOI: 10.1016/j.cct.2010.08.002] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT01197443 {published data only}
- Acronym: PAAC. Ongoing study Study start date: November 2010 Study completion date: July 2015.
NCT01546727 {published data only}
- Acronym: Behavioral Treatment for Obese Preschoolers (LAUNCH). Ongoing study Study start date: March 2012 Study completion date: November 2016.
NCT01552642 {published data only}
- Acronym: none. Ongoing study Study start date: February 2013 Study completion date: August 2015.
NCT01792531 {published data only}
- Acronym: More and Less study (M+L). Ongoing study Study start date: January 2013 Study completion date: December 2017.
NCT02373670 {published data only}
- Acronym: none. Ongoing study Study start date: January 2015 Study completion date: June 2015.
Önnerfält 2012 {published data only}
- Önnerfält J, Erlandsson LK, Orban K, Broberg M, Helgason C, Thorngren‐Jerneck K. A family‐based intervention targeting parents of preschool children with overweight and obesity: conceptual framework and study design of LOOPS ‐ Lund Overweight and Obesity Preschool Study. BMC Public Health 2012;12:879. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Beller 2013
- Beller EM, Chen JK, Wang UL, Glasziou PP. Are systematic reviews up‐to‐date at the time of publication?. Systematic Reviews 2013;2(1):36. [2046‐4053: (Electronic)] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bocca 2013
- Bocca G, Ongering EC, Stolk RP, Sauer PJ. Insulin resistance and cardiovascular risk factors in 3‐ to 5‐year old overweight or obese children. Hormone Research in Paediatrics 2013;80(3):201‐6. [DOI] [PubMed] [Google Scholar]
Clark 2007
- Clark HR, Goyder E, Bissell P, Blank L, Peters J. How do parents' child feeding behaviours influence child weight? Implications for childhood obesity policy. Journal of Public Health 2007;29:132‐41. [DOI] [PubMed] [Google Scholar]
CMO 2012
- Chief Medical Officer. Annual report of the Chief Medical Officer: surveillance volume, 2012: on the State of the Public's Health. www.gov.uk/government/publications/chief‐medical‐officer‐annualreport‐surveillance‐volume‐2012 (accessed 30 March 2015).
Cole 2000
- Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 2000;320(7244):1240‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cole 2012
- Cole TJ, Lobstein T. Extended international (IOTF) body mass index cut‐offs for thinness, overweight and obesity. Pediatric Obesity 2012;7(4):284‐94. [DOI] [PubMed] [Google Scholar]
Daniels 2009
- Daniels SR. Complications of obesity in children and adolescents. International Journal of Obesity 2009;33(Suppl 1):S60‐5. [DOI] [PubMed] [Google Scholar]
de Onis 2010
- Onis M, Blössner M. Borghi E. Global prevalence and trends of overweight and obesity among preschool children. American Journal of Clinical Nutrition 2010;92(5):1257‐64. [DOI] [PubMed] [Google Scholar]
Eady 2008
- Eady AM, Wilczynski NL, Haynes RB. PsycINFO search strategies identified methodologically sound therapy studies and review articles for use by clinicians and researchers. Journal of Clinical Epidemiology 2008;61(1):34‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Egan 2013
- Egan K, Ettinger A, Bracken M. Childhood body mass index and subsequent physician‐diagnosed asthma: a systematic review and meta‐analysis of prospective cohort studies. BMC Pediatrics 2013;13(1):121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Egbewale 2014
- Egbewale BE, Lewis M, Sim J. Bias, precision and statistical power of analysis of covariance in the analysis of randomized trials with baseline imbalance: a simulation study. BMC Medical Research Methodology 2014;14:49:49. [DOI: 10.1186/1471-2288-14-49] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ells 2015
- Ells LJ, Hancock C, Copley VR, Mead E, Dinsdale H, Kinra S, et al. Prevalence of severe childhood obesity in England: 2006‐2013. Archives of Disease in Childhood 2015;100(7):631‐6. [DOI] [PubMed] [Google Scholar]
Ewald 2014
- Ewald H, Kirby J, Rees K, Robertson W. Parent‐only interventions in the treatment of childhood obesity: a systematic review of randomized controlled trials. Journal of Public Health 2014;36(3):476‐89. [DOI] [PubMed] [Google Scholar]
Faith 2012
- Faith MS, Horn L, Appel LJ, Burke LE, Carson JAS, Franch HA, et al. AHA scientific statement: evaluating parents and adult caregivers as "Agents of Change" for treating obese children: evidence for parent behavior change strategies and research gaps: a scientific statement from the American Heart Association. Circulation 2012;125:1186‐207. [DOI] [PubMed] [Google Scholar]
Follman 1992
- Follmann D, Elliot P, Suh I, Cutler J. Variance imputation for overviews of clinical trials with continuous response. Journal of Clinical Epidemiology 1992;45(7):769‐73. [DOI] [PubMed] [Google Scholar]
Freedman 2006
- Freedman DS, Khan LK, Serdula MK, Ogden CL, Dietz WH. Racial and ethnic differences in secular trends for childhood BMI, weight, and height. Obesity 2006;14(2):301‐8. [DOI] [PubMed] [Google Scholar]
Glenny 1997
- Glenny AM, O'Meara S, Melville A, Sheldon TA, Wilson C. The treatment and prevention of obesity: a systematic review of the literature. International Journal of Obesity and Related Metabolic Disorders 1997;21(9):715‐37. [DOI] [PubMed] [Google Scholar]
Golan 2004
- Golan M, Crow S. Targetting parents exclusively in the treatment of childhood obesity: long‐term results. Obesity Research 2004;12:357‐61. [DOI] [PubMed] [Google Scholar]
Griffiths 2010
- Griffiths LJ, Parsons TJ, Hill AJ. Self‐esteem and quality of life in obese children and adolescents: a systematic review. International Journal of Pediatric Obesity 2010;5(4):282‐304. [DOI] [PubMed] [Google Scholar]
Gustafson 2006
- Gustafson SL, Rhodes RE. Parental correlates of physical activity in children and early adolescents. Sports Medicine 2006;36:79‐97. [DOI] [PubMed] [Google Scholar]
Halliday 2014
- Halliday JA, Palma CL, Mellor D, Green J, Renzaho AMN. The relationship between family functioning and child and adolescent overweight and obesity: a systematic review. International Journal of Obesity 2014;38:480‐93. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2009
- Higgins JPT, Thompson SG, Spiegelhalter DJ. A re‐evaluation of random‐effects meta‐analysis. Journal of the Royal Statistical Society. Series A (Statistics in Society) 2009;172(1):137‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hróbjartsson 2013
- Hróbjartsson A, Thomsen AS, Emanuelsson F, Tendal B, Hilden J, Boutron I, et al. Observer bias in randomized clinical trials with measurement scale outcomes: a systematic review of trials with both blinded and nonblinded assessors. Canadian Medical Association Journal 2013;185(4):E201‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
HSCIC 2015
- Health and Social Care Information Centre. National Child Measurement Programme ‐ England, 2012‐13 school year. www.hscic.gov.uk/catalogue/PUB13115 (accessed 30 March 2015).
Jang 2015
- Jang M, Chao A, Whittemore R. Evaluating intervention programs targeting parents to manage childhood overweight and obesity: a systematic review using the RE‐AIM framework. Journal of Pediatric Nursing 2015;30(6):877‐87. [DOI: 10.1016/j.pedn.2015.05.004.] [DOI] [PubMed] [Google Scholar]
Jull 2013
- Jull A, Chen R. Parent‐only vs. parent‐child (family‐focused) approaches for weight loss in obese and overweight children: a systematic review and meta‐analysis. Obesity Reviews 2013;14:761‐8. [DOI] [PubMed] [Google Scholar]
Kelly 2013
- Kelly AS, Barlow SE, Rao G, Inge TH, Hayman LL, Steinberger J, et al. Severe obesity in children and adolescents: identification, associated health risks, and treatment approaches: a scientific statement from the American Heart Association. Circulation 2013;128(15):1689‐712. [DOI] [PubMed] [Google Scholar]
Kirkham 2010
- Kirkham JJ, Dwan KM, Altman DG, Gamble C, Dodd S, Smyth R, et al. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ 2010;340:c365. [DOI: 10.1136/bmj.c365] [DOI] [PubMed] [Google Scholar]
Knai 2012
- Knai C, Lobstein T, Darmon N, Rutter H, McKee M. Socioeconomic patterning of childhood overweight status in Europe. International Journal of Environmental Research and Public Health 2012;9(4):1472‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leclercq 2013
- Leclercq E, Leeflang MM, Dalen EC, Kremer LC. Validation of search filters for identifying pediatric studies in PubMed. Journal of Pediatrics 2013;162(3):629‐34. [DOI] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic and meta‐analyses of studies that evaluate interventions: explanation and elaboration. PLoS Medicine 2009;6(7):1‐28. [DOI: 10.1371/journal.pmed.1000100] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lobstein 2004
- Lobstein T, Baur L, Uauy R. Obesity in children and young people: a crisis in public health. Obesity Reviews 2004;5(Suppl 1):4‐104. [DOI] [PubMed] [Google Scholar]
Meader 2014
- Meader N, King K, Llewellyn A, Norman G, Brown J, Rodgers M, et al. A checklist designed to aid consistency and reproducibility of GRADE assessments: development and pilot validation. Systemic Reviews 2014;3:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Narang 2012
- Narang I, Mathew JL. Childhood obesity and obstructive sleep apnea. Journal of Nutrition and Metabolism 2012;2012:134202. [DOI: 10.1155/2012/134202; PUBMED: 22957216] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ng 2014
- Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980‐2013: a systematic analysis of the Global Burden of Disease Study 2013. Lancet 2014;384(9945):766‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2013
- National Institute for Health and Care Excellence. Managing overweight and obesity among children and young people: lifestyle weight management services. NICE Guidelines PH47, 2013. www.nice.org.uk/guidance/ph47 (accessed 15 December 2015).
Noo NHS 2011
- National Obesity Observatory on behalf of the Public Health Observatories in England. A simple guide to classifying body mass index in children, 2011. www.noo.org.uk/uploads/doc/vid_11601_A_simple_guide_to_classifying_BMI_in_children.pdf (accessed 3 November 2015).
Ogden 2006
- Ogden J, Reynolds R, Smith A. Expanding the concept of parental control: a role for overt and covert control in children's snacking behaviour?. Appetite 2006;47:100‐6. [DOI] [PubMed] [Google Scholar]
Olds 2011
- Olds T, Maher C, Zumin S, Péneau S, Lioret S, Castetbon K, et al. Evidence that the prevalence of childhood overweight is plateauing: data from nine countries. International Journal of Pediatric Obesity 2011;6(5‐6):342‐60. [DOI] [PubMed] [Google Scholar]
Oude Luttikhuis 2009
- Oude Luttikhuis H, Baur L, Jansen H, Shrewbury VA, O'Malley C, Stolk RP, et al. Interventions for treating obesity in children. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD001872.pub2] [DOI] [PubMed] [Google Scholar]
Parsons 1999
- Parsons TJ, Power C, Logan S, Summerbell CD. Childhood predictors of adult obesity: a systematic review. International Journal of Obesity 1999;23(Suppl 8):S1‐107. [PubMed] [Google Scholar]
Paulis 2014
- Paulis WD, Silva S, Koes BW, Middelkoop M. Overweight and obesity are associated with musculoskeletal complaints as early as childhood: a systematic review. Obesity Reviews 2014;15(1):52‐67. [DOI] [PubMed] [Google Scholar]
Puhl 2007
- Puhl RM, Latner JD. Stigma, obesity, and the health of the nation's children. Psychology Bulletin 2007;133(4):557‐80. [DOI] [PubMed] [Google Scholar]
Rajput 2014
- Rajput N, Tuohy P, Mishra S, Smith A, Taylor B. Overweight and obesity in 4‐5‐year‐old children in New Zealand: results from the first 4 years (2009‐2012) of the B4 School Check programme. Journal of Paediatrics and Child Health 2014;51(3):334‐43. [DOI] [PubMed] [Google Scholar]
Reilly 2003
- Reilly JJ, Methven E, McDowell ZC, Hacking B, Alexander D, Stewart L, et al. Health consequences of obesity. Archives of Diseases in Childhood 2003;88(9):748‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reilly 2011
- Reilly JJ, Kelly J. Long‐term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: systematic review. International Journal of Obesity 2011;35(7):891‐8. [DOI] [PubMed] [Google Scholar]
Riley 2011
- Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta‐analyses. BMJ 2011;342:d549. [DOI] [PubMed] [Google Scholar]
Rokholm 2010
- Rokholm B, Baker JL, Sørenson TI. The levelling off of the obesity epidemic since the year 1999 ‐ a review of evidence and perspectives. Obesity Reviews 2010;11(12):835‐46. [DOI] [PubMed] [Google Scholar]
Shrewsbury 2008
- Shrewsbury V, Wardle J. Socioeconomic status and adiposity in childhood: a systematic review of cross‐sectional studies 1990‐2005. Obesity (Silver Spring) 2008;16(2):275‐84. [DOI] [PubMed] [Google Scholar]
Singh 2008
- Singh AS, Mulder C, Twisk JW, vanMechelen W, Chinapaw MJ. Tracking of childhood overweight into adulthood: a systematic review of the literature. Obesity Reviews 2008;9(5):474‐88. [DOI] [PubMed] [Google Scholar]
Skinner 2014
- Skinner AC, Skelton JA. Prevalence and trends in obesity and severe obesity among children in the United States, 1999‐2012. JAMA Pediatrics 2014;168(6):561‐6. [DOI] [PubMed] [Google Scholar]
Sleddens 2011
- Sleddens EF, Gerards SM, Thijs C, Vries NK, Kremers SP. General parenting, childhood overweight and obesity‐inducing behaviors: a review. International Journal of Pediatric Obesity 2011;6:e12‐27. [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
Tang‐Peronard 2008
- Tang‐Peronard JL, Heitmann BL. Stigmatization of obese children and adolescents, the importance of gender. Obesity Reviews 2008;9(6):522‐34. [DOI] [PubMed] [Google Scholar]
Upton 2012
- Upton D, Upton P, Bold J, Peters DM. Regional evaluation of weight management programmes for children and families, 2012. www.obesitywm.org.uk/resources/Worcester_Report_Fina.pdf (accessed 15 December 2015).
Wang 2012
- Wang Y, Lim H. The global childhood obesity epidemic and the association between socio‐economic status and childhood obesity. International Review of Psychiatry 2012;24(3):176‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wansink 2006
- Wansink B. Nutritional gatekeepers and the 72% solution. Journal of the American Dietetic Association 2006;106:1324‐7. [DOI] [PubMed] [Google Scholar]
Whitaker 1997
- Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH. Predicting obesity in young adulthood from childhood and parental obesity. New England Journal of Medicine 1997;337(13):869‐73. [DOI] [PubMed] [Google Scholar]
WHO 2015a
- World Health Organization. Fact sheet on overweight and obesity. www.who.int/mediacentre/factsheets/fs311/en (accessed 30 March 2015).
WHO 2015b
- World Health Organization. The WHO Child Growth Standards. www.who.int/childgrowth/publications/technical.report.pub/en/ (accessed 30 March 2015).
Wong 2006a
- Wong SS, Wilczynski NL, Haynes RB. Developing optimal search strategies for detecting clinically sound treatment studies in EMBASE. Journal of the Medical Library Association 2006;94(1):41‐7. [PMC free article] [PubMed] [Google Scholar]
Wong 2006b
- Wong SS, Wilczynski NL, Haynes RB. Optimal CINAHL search strategies for identifying therapy studies and review articles. Journal of Nursing Scholarship 2006;38(2):194‐9. [DOI] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Juni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ 2008;336(7644):601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Summerbell 2003
- Summerbell CD, Ashton V, Campbell KJ, Edmunds L, Kelly S, Waters E. Interventions for treating obesity in children. Cochrane Database of Systematic Reviews 2003, Issue 3. [DOI: 10.1002/14651858.CD001872] [DOI] [PubMed] [Google Scholar]


