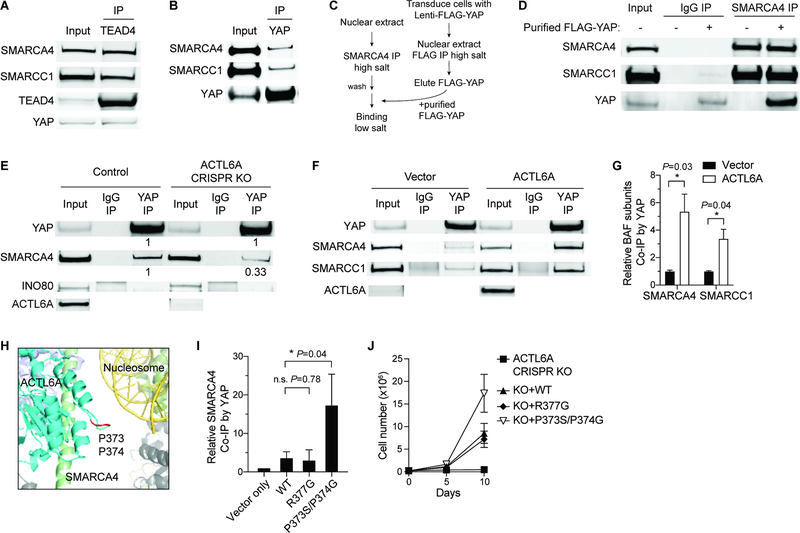
Figure 5. ACTL6A promotes the direct binding of TEAD-YAP to BAF complexes.
(A-B) Co-IP experiments by TEAD4 (A) and YAP (B) antibodies using nuclear extracts from FaDu SCC cells.
(C) Workflow for in vitro binding experiments. BAF complexes and FLAG-YAP were purified separately under high-salt buffer conditions, and then co-incubated under low-salt buffer condition for in vitro binding examination.
(D) In vitro binding of purified FLAG-YAP and BAF complexes by SMARCA4 antibody-IP.
(E) Co-IP experiments by YAP and IgG antibodies showing decreased binding of SMARCA4 with YAP in ACTL6A CRISPR-knockout (KO) FaDu SCC cells. Quantifications normalized to control. INO80: INO80-complex subunit.
(F) Co-IP experiments by YAP and IgG antibodies in primary human keratinocytes overexpressing ACTL6A and vector-control.
(G) Quantifications for (F) showing increased binding of YAP and BAF subunits upon ACTL6A overexpression. Normalized to vector control. n=3 experiments. Mean ± SEM. *P < 0.05.
(H) The human BAF complex cryo-EM structure (PDB: 6LTJ) showing the position of ACTL6A P373/P374 residues (marked in red) in the nucleosome-bound BAF complex. Cyan: ACTL6A. Red: P373/P374 residues of ACTL6A. Green: HSA domain of SMARCA4. Yellow: Nucleosomal DNA. Olive: histone octamer.
(I) Quantifications for co-IP experiments by YAP antibodies in keratinocytes overexpressing WT, R377G and P373S/P374G ACTL6A. Normalized to vector control. n=3 experiments. Mean ± SEM. *P < 0.05. n.s.: not significant.
(J) Growth curves of FaDu SCC cells transduced with lentiviral constructs for ACTL6A CRISPR-KO and simultaneously reconstituted with KO-resistant WT, R377G, P373S/P374G ACTL6A, or vector-control. n=3 experiments. Error bars indicate SD.