Figure S1. Pharmacological properties of FN104.
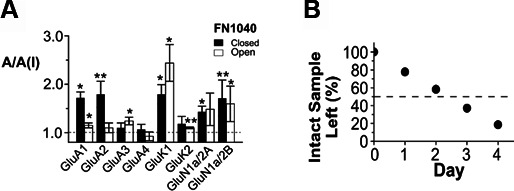
(A) The selectivity of the full-length aptamer FN1040. The selectivity was measured in the A/A(I) value using the same aptamer concentration (2 μM) against the subunit and channel types. The glutamate concentrations used for closed-channel (solid columns) and open-channel (hollow column) conformation (in mM) were 0.05 and 3 for GluA1, 0.1 and 3 for GluA2-GluA4, 0.05 and 3 for GluK1 and GluK2, and 0.02 and 0.05 for GluN1a/2A and GluN1a/2B, respectively. One-sample two-tailed t tests on these data showed the mean values of A/A(I) on closed-channel conformation of GluA2Q, GluK1, GluN1a/2A, and GluN1a/2B, and on open-channel form of GluA3, GluK1, GluK2, and GluN1a/2B were statistically significantly. Specifically, one-sample two-tailed t tests were performed on each column with H0: µ = µ0 = 1, 1 being the theoretical value of no inhibition (P ≤ 0.5). The significance of the t test for each column was labeled as the double asterisk (P ≤ 0.1) or the single asterisk (P ≤ 0.5). A one-way ANOVA analysis combing with post hoc Tukey’s test indicated that, on the closed-channel conformation, the mean A/A(I) value difference was significant (P ≤ 0.5) only between GluA2 and GluA3, or between GluA2 and GluA4. (B) In vitro stability of FN1040 in rat cerebrospinal fluid. The FN1040 samples taken at each of the time point were examined on an 8 M urea PAGE. The band intensity of the intact or the full-length RNA was quantified and normalized to the initial sample in the lane labeled as “0” (time 0 means that the sample contained no medium or no ribonucleases). The t1/2 of FN1040 was estimated to be ∼2.5 d. The data shown in this figure were originally published in Huang et al (2017) ACS Chem Neurosci 8: 2437–2445 and are reprinted (adapted) with the permission from the journal. Copyright (2017) American Chemical Society.
