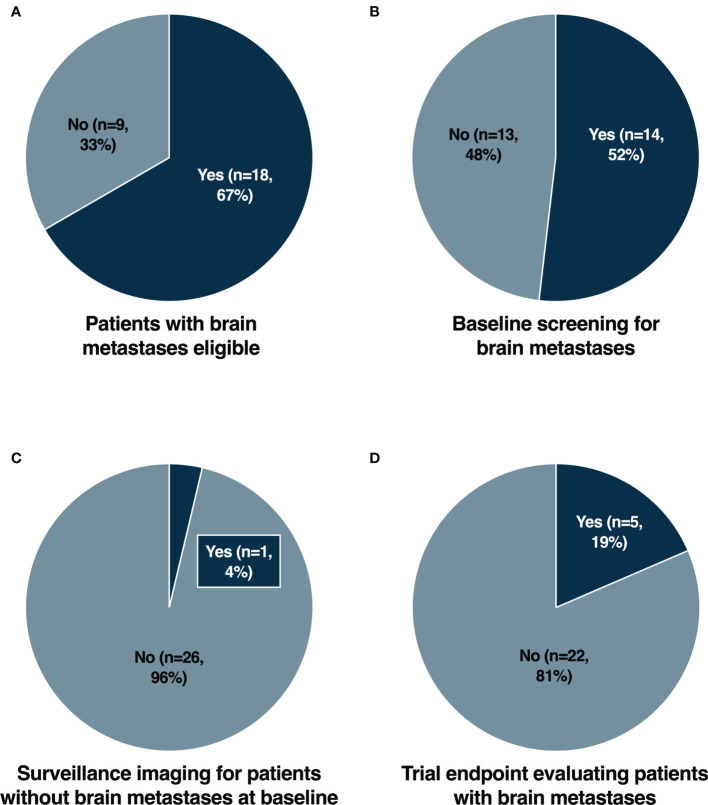
Figure 1.
Eligibility for patients with brain metastases (A), baseline screening for brain metastases (B), surveillance imaging for patients without brain metastases at baseline (C) and trial endpoint evaluating patients with brain metastases (D) in registrational trials for newly FDA approved cancer therapies from 2018-2020.