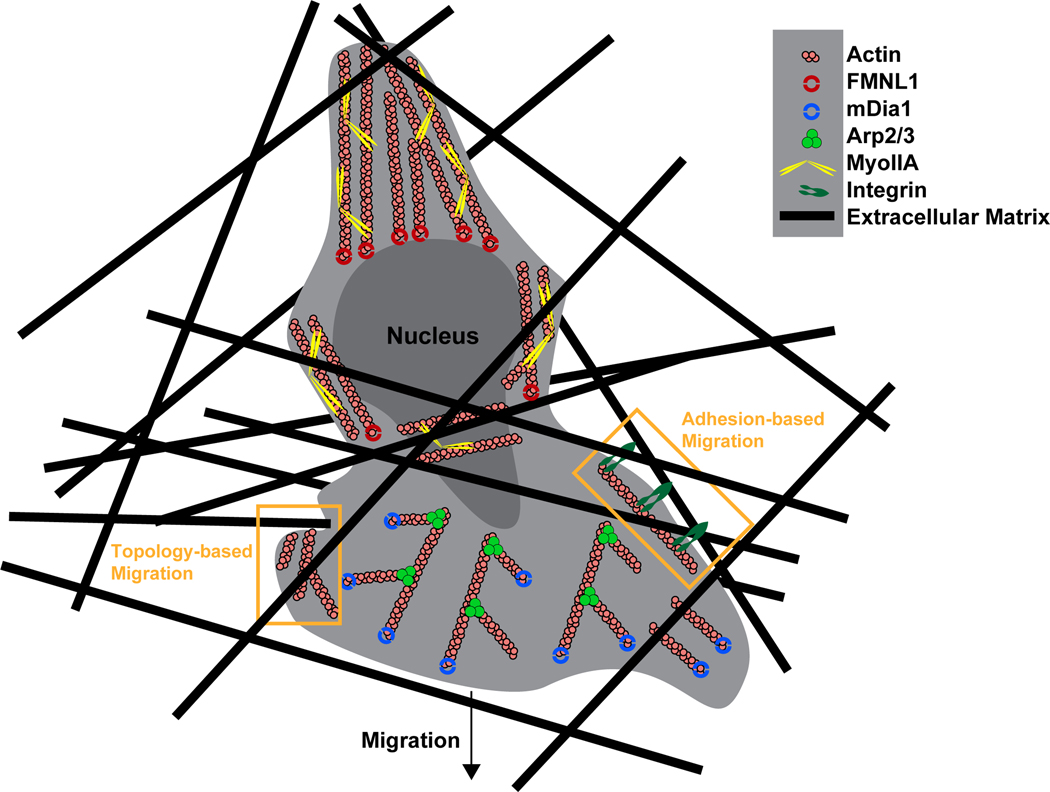
Figure 4. Model of the actin polymerization machinery involved in T cell interstitial migration.
During interstitial migration actin polymerization by Arp2/3 and mDia1 at the leading edge of the cell promote expansion of the leading edge and formation of protrusions to facilitate environmental sensing. Depending on the tissue environment, actin polymerization can enable motility in the absence of adhesions, in that actin structures may create a sufficient frictional interface by pushing directly against the surroundings, such as the ECM or other cells (topology-based migration). However, in constrictive ECM-dense environments such as the skin, integrin adhesion can play an important role in interstitial migration, as greater force transfer may be required to enable migration. During this adhesion-based migration, adhesion receptors such as chemokine-activated integrins, which are coupled to actin structures, can bind to the ECM, serving as a clutch to engage the ‘motor’ of actin polymerization, and provide the traction force necessary to move cells forward. At the rear of the cell, actin filament contraction by MyoIIA allows cellular and nucleus deformation for navigating small openings. Similar to its role in diapedesis, FMNL1 is likely also involved in facilitating squeezing of the nucleus through constrictive barriers within tissues.