Introduction
Acute pericarditis is a rare complication of mRNA SARS-CoV-2 vaccination [1, 2] and we describe a patient who initially presented with new-onset atrial fibrillation.
Clinical presentation
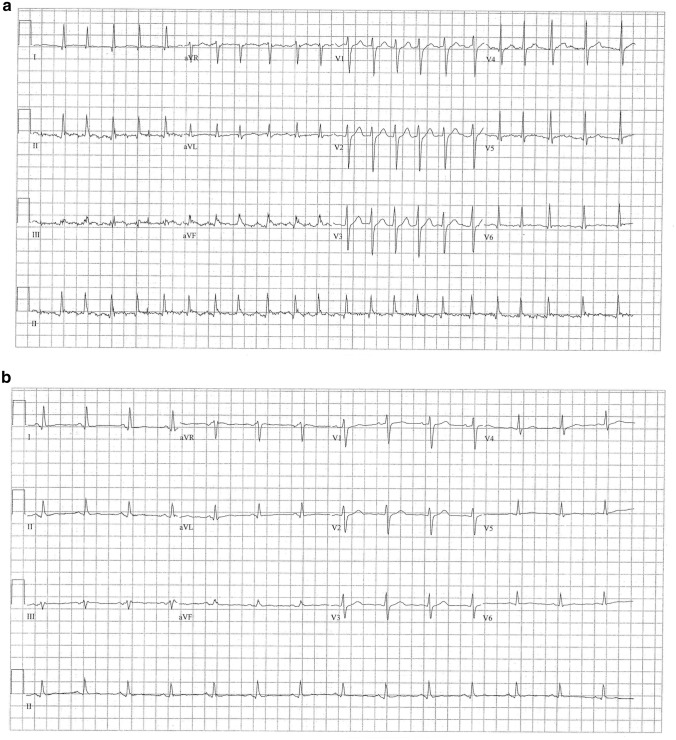
A healthy 49-year-old male without prescription medications presented to the emergency department with 30 min of palpitations. He had mild dull non-positional, non-radiating chest discomfort but was not short of breath and had no fever. Eight days previously, he had received his second mRNA-1273 (Moderna) SARS CoV-2 vaccination. His vital signs included a heart rate of 110–125 beats per minute, but his blood pressure and respiratory rate were normal. His examination was only noteworthy for an irregularly irregular pulse. Electrocardiogram (ECG) confirmed atrial fibrillation without any segment elevations or depressions. (Fig. 1 left panel) His white blood cell count was 14.5 × 103 cc / L and all other tests including brain natriuretic peptide, troponin, thyroid-stimulating hormone, and chest radiography were normal. After discussion and informed consent, he was electrically converted to normal sinus rhythm; the post-procedure ECG was normal without any segment deviations or abnormal intervals. He was discharged home with a prescription for rivaroxaban and an outpatient cardiology appointment in 3 weeks.
Fig. 1.
a Initial electrocardiogram at index visit demonstrating atrial fibrillation with rapid ventricular response. b Initial electrocardiogram at third visit demonstrating normal sinus rhythm with decreased amplitude
Two days later, he returned with 1 hour of atrial fibrillation with normal repeat investigations but failed three attempts at electrical cardioversion. He was converted uneventfully to normal sinus rhythm with procainamide, and the final ECG was normal. One week after the index visit, he re-presented again with a few days’ of increasing sharp chest pain relieved with sitting up, as well as worsening shortness of breath aggravated with exertion. The emergency physician performed a bedside ultrasound that demonstrated normal contractility and a moderate pericardial effusion. His neck veins were not distended. His C-reactive protein was elevated at 97.6 mg / L and d-dimer was 2173 ng / mL; the ECG demonstrated normal sinus rhythm but decreased amplitude. (Fig. 1 right panel) Computed tomography confirmed a pericardial effusion but no other pathology. The admitting cardiologist reported a friction rub, and along with his characteristic pain, the new effusion, the elevated C-reactive protein, the timing of his symptoms, as well as the lack of any other inciting traumatic, procedural, infectious, or inflammatory cause, he was diagnosed with pericarditis related to mRNA-1273 SARS CoV-2 vaccination. He was admitted overnight, and the cardiology team halted anticoagulation, initiated non-steroidal anti-inflammatories, and discharged him uneventfully the next morning. At two weeks, he had mild dyspnea and a follow-up echocardiogram demonstrated a much smaller effusion. At 6- and 12-week follow-ups, he was symptom-free.
Discussion
Acute pericarditis is an inflammation of the pericardium and is distinguished by characteristic sharp chest pain classically worse with recumbency and relieved with sitting up. The illness ranges from trivial self-limited symptoms to rare life-threatening cases complicated by pericardial effusion and tamponade physiology. Electrocardiographic findings include diffuse ST-segment elevation (as opposed to regional ST-segment elevation in myocardial infarction, [STEMI] or confined to anterior leads in benign early repolarization [BER]); concave ST-segment elevation (as with BER, but as opposed to convex elevation in STEMI); and the ST-segment: T wave height ratio (ST/T ratio) is typically > 0.25 in pericarditis and < 0.25 in BER. However, patients with pericarditis may also have a normal ECG.
Pericarditis is a recognized complication of COVID-19 mRNA vaccines, and widespread vaccine availability has led to a recent increase in the diagnosis of pericarditis [1]. This illness typically occurs 1–2 weeks post vaccination in patients with a median age of 50. Given the small number of cases, the pathophysiology is so far unknown, but proposed mechanisms include hypersensitivity to vaccine constituents, an excessive inflammatory reaction, or an inappropriate immune response [2].
Canadian clinical practice guidelines suggest making a diagnosis of mRNA-associated pericarditis on the following: symptom onset within a week of mRNA vaccination, chest pain, dyspnea, or syncope; ECG suggesting pericarditis (diffuse ST segment elevation, high-grade heart block, or PR-segment depression); elevated cardiac and inflammatory biomarkers; and presence of pericardial effusion [3]. In complex cases, cardiac magnetic resonance imaging or endocardial biopsy may be warranted [3]. The majority of diagnoses are considered probable and founded on history, physical examination, biomarkers, and non-invasive imaging. However, confirmation requires biopsy and histologic assessment [3]. The illness is reportable to Public Health [3]. Importantly, most patients can be safely discharged home without incident; even patients who are admitted typically only remain for a single day [1]. Treatment is largely supportive, and no specific management is currently recommended [3]. It is presently unclear whether such patients should avoid an mRNA vaccination or opt for a non-mRNA vaccination instead [3]. However, given that SARS-CoV2 infection (COVID-19) can cause myocarditis and pericarditis at far higher rates than mRNA vaccines, as well as heart failure, arrhythmias, myocardial infarction, and thromboembolic events, the risks of infection must be weighed against the risks of vaccination [3].
It is exceedingly rare for post-viral pericarditis to present with atrial fibrillation: in 822 consecutive patients with pericarditis recruited at two European hospitals over 8 years, 34 (4.3%) developed atrial fibrillation within 24 h of their diagnosis of pericarditis, and no patients were recorded to have atrial fibrillation prior to their pericarditis diagnosis. In addition, patients who developed atrial fibrillation were significantly older than those who did not, and almost all converted spontaneously to sinus rhythm within 24 h [4].
While mRNA vaccinations have slightly increased the risk of pericarditis [1] above baseline, there are no corresponding population-level reports of increased atrial fibrillation rates due to mRNA vaccinations. Only one patient has been reported with post-vaccination atrial fibrillation—a 30-year-old male with Marfan’s syndrome several days post coronary artery bypass grafting who received mRNA vaccination and developed atrial fibrillation a week later—[2] a situation very different than our patient. Since post-vaccination atrial fibrillation appears to be exceedingly rare, we cannot endorse that all patients with new or deteriorating atrial fibrillation require extensive investigations for potential underlying causes. However, in new atrial fibrillation patients who have had a recent mRNA vaccination, and especially in those who present with chest discomfort or dyspnea, cardiac inflammation including myocarditis and pericarditis [1] are both potential diagnoses with a small but noteworthy possibility of serious complications. Physicians should consider physical examination maneuvers, such as auscultation for a friction rub, careful assessment of neck veins, or ascertainment of pulsus paradoxus. Importantly, physicians can attempt low-barrier diagnostics such as a bedside ultrasound [5]. These tests are rapid, inexpensive, and can be performed nearly any environment with minimal patient inconvenience.
Funding
No funding was secured for the manuscript.
Declarations
Conflict of interest
None of the authors have a conflict of interest to declare.
References
- 1.Diaz GA, Parsons GT, Gering SK, et al. Myocarditis and pericarditis after vaccination for Covid-19. JAMA. 2020;326:1210–1212. doi: 10.1001/jama.2021.13443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li K, Huang B, Ji T, et al. A postoperative man with Marfan Syndrome with palpitations and chest pain after receiving the SARS-CoV-2 vaccine. Inf Drug Resist. 2021;14:2953–2956. doi: 10.2147/IDR.S323280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luk A, Clarke B, Dahdah N, et al. Myocarditis and pericarditis after Covid-19 mRNA vaccination: practical considerations for care providers. Can J Cardiol. 2021;37:1629–1634. doi: 10.1016/j.cjca.2021.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Imazio M, Lazaros G, Picardi E, et al. Incidence and prognostic significance of new-onset atrial fibrillation / flutter in acute pericarditis. Heart. 2014;101:1463. doi: 10.1136/heartjnl-2014-307398. [DOI] [PubMed] [Google Scholar]
- 5.Nagdev A, Stone M. Point of care ultrasound evaluation of pericardial effusions: does this patient have cardiac tamponade? Resuscitation. 2011;82:671–673. doi: 10.1016/j.resuscitation.2011.02.004. [DOI] [PubMed] [Google Scholar]