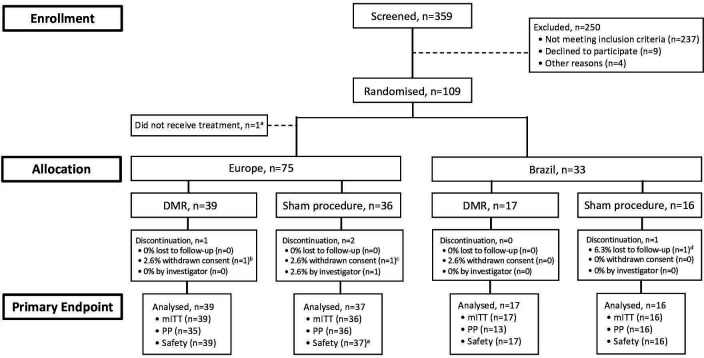
Figure 1.
Patient disposition. Between 1 March 2017, and 15 December 2018, 109 of 359 patients assessed for eligibility were randomised. One patient at a European study site did not receive treatment due to oesophageal varicesa; therefore, the modified intention-to-treat (mITT) population included 108 patients—75 in Europe (39 to duodenal mucosal resurfacing (DMR) and 36 to sham) and 33 in Brazil (17 to DMR and 16 to sham). Prespecified assessments of normality and homogeneity (see the Methods section for details) revealed that the European and Brazilian populations were not poolable. Therefore, all efficacy analyses were stratified into two populations (Europe and Brazil). aThe one patient who withdrew and did not receive treatment was followed for safety, but not efficacy. Last completed visit was at week 4a, week 21 (phone call)b, and day 7 (phone call)d. PP, per protocol.