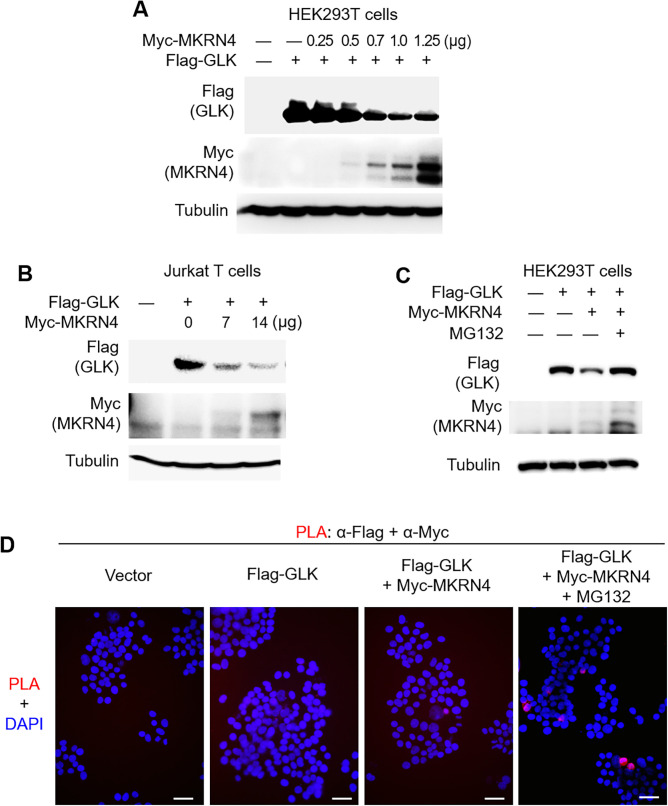
Figure 5.
The novel E3 ligase MKRN4 induces proteasomal degradation of GLK. (A) Immunoblotting of Flag-tagged GLK (anti-FLAG), Myc-tagged MKRN4 (anti-MYC) and tubulin proteins from Jurkat T cells cotransfected with Flag-GLK plus increasing amounts of Myc-MKRN4 plasmids. (B) Immunoblotting of Flag-tagged GLK (anti-FLAG), Myc-tagged MKRN4 (anti-MYC) and tubulin proteins from HEK293T cells cotransfected with Flag-GLK plus increasing amounts of Myc-MKRN4 plasmids. (C) Immunoblotting of Flag-tagged GLK (anti-FLAG), Myc-tagged MKRN4 (anti-MYC) and tubulin proteins from HEK293T cells cotransfected with Flag-GLK plus Myc-MKRN4 plasmids. Cells were treated with 25 µM MG132 for 2 hours before being harvested. (D) In situ PLA assays of the interaction between Myc-tagged MKRN4 and Flag-tagged GLK proteins in HEK293T cells. Cells were treated with 25 µM MG132 for 2 hours before being harvested. Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI). Imaging was detected by Leica DM2500 upright fluorescence microscope. Original magnification, ×200. scale bars, 50 µm. PLA, proximity ligation assay.