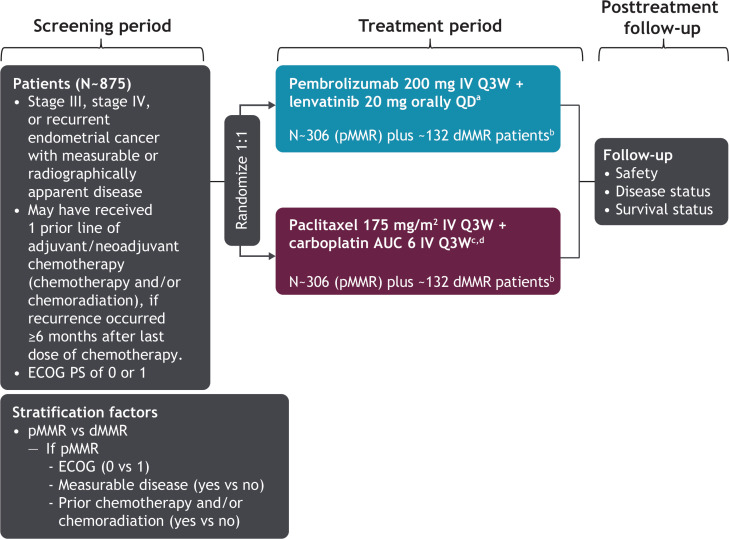
Figure 1.
ENGOT-en9/LEAP-001 study design. aTreat until disease progression or unacceptable toxicity. Pembrolizumab must be stopped after 35 cycles, but lenvatinib may continue after stopping pembrolizumab. bStudy will be fully enrolled when 612 patients with mismatch repair-proficient (pMMR) tumors and ~263 patients with mismatch repair-deficient (dMMR) tumors are recruited. cA lower starting dose of paclitaxel (135 mg/m2) and carboplatin (AUC 5 mg/mL/min) may be administered to patients at risk of developing toxicities due to previous pelvic/spine radiation. An AUC of 5 mg/mL/min dose of carboplatin may be administered in accordance with local practice. dPatients may receive up to seven cycles of paclitaxel/carboplatin; however, chemotherapy treatment beyond seven cycles may be permitted (with the sponsors’ approval) for patients who continue to derive clinical benefit. AUC, area under the curve (unit, mg/mL/min); ECOG, Eastern Cooperative Oncology Group; IV, intravenous; PS, performance status; QD, once daily; Q3W, every 3 weeks.