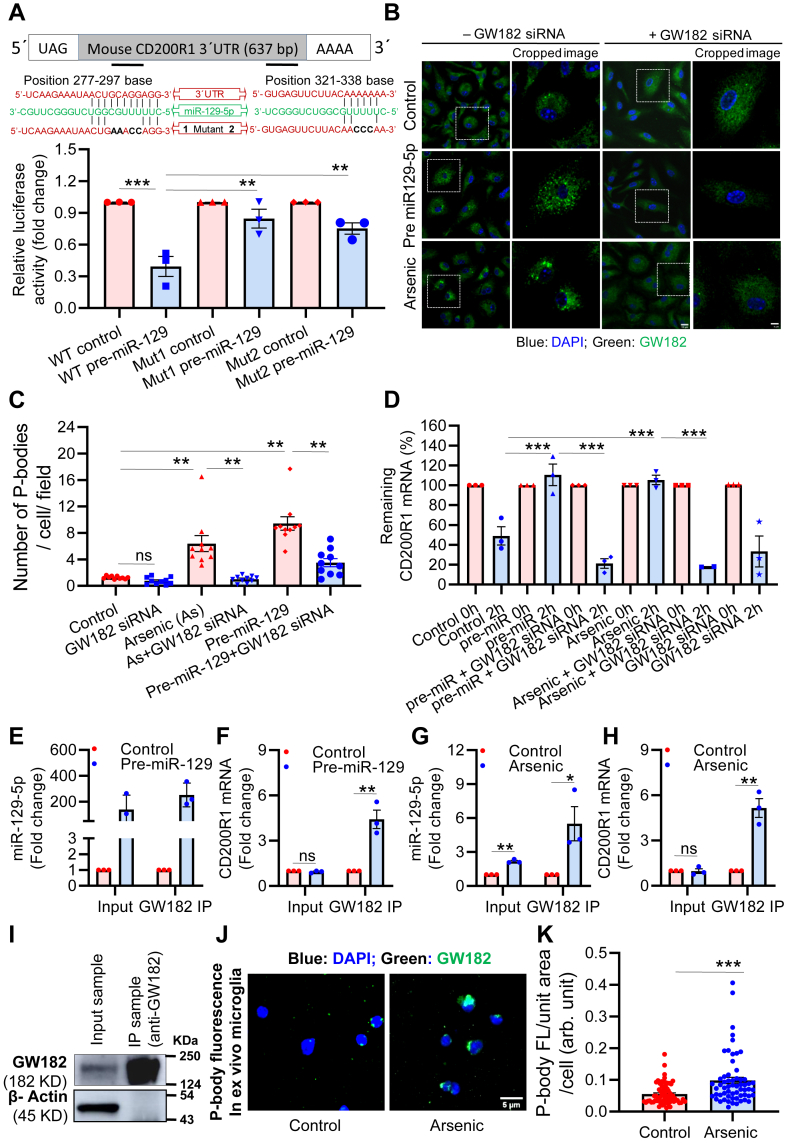
Figure 3.
MiR-129-5p guides CD200R1 mRNA to p-bodies.A, luciferase reporter assay using mouse CD200R1 3′-UTR constructs (pMIR-WT, pMIR-Mut1 & pMIR-Mut2) cloned in pMIR-REPORT vector confirmed that both the predicted binding sites are involved in miR-129-5p-mediated repression of CD200R1 (n = 3). B and C, P-bodies were stained with GW182 antibody in primary neonatal microglia following pre-miR-129 and arsenic exposure for 72 h. It showed increased puncta formation in the cytoplasm. Scale bar: 10 μm for uncropped images and 4 μm for the cropped images. D, microglia were treated with pre-miR-129 and arsenic for 72 h followed by actinomycin D (5 μg/ml) treatment for 0, 2 h. Cells were harvested, RNA isolated, and run for real-time PCR to detect CD200R1. Pre-miR-129 and arsenic protected CD200R1 mRNA from degradation. To detect the presence of miR-129-5p and CD200R1 mRNA in p-bodies, GW182 and pre-miR-129 were overexpressed in BV2 cells (n = 3) and the levels of (E) miR-129-5p and (F) CD200R1 mRNA were measured in GW182 immunoprecipitated (GW182 IP) samples. Similarly, GW182 overexpressed BV2 cells were treated with arsenic (n = 3) and level of (G) miR-129-5p, and (H) CD200R1 mRNA were measured in GW182 IP samples. Levels of both miR-129-5p and CD200R1 mRNA in the GW182 IP samples were found to be significantly high (n = 3). I, to check the contamination-free IP of GW182, the GW182 protein was immunoprecipitated using an anti-GW182 antibody and processed for WB analysis. Sixty microgram lysate (7.5% of input for IP [800 μg]) loaded in lane-1 and 12 μl (60% of the total IP elution volume [20 μl]) loaded in lane-2. The absence of the β-actin band in IP lane shows the contamination free IP of GW182 (n = 2). Formation of P-body in vivo was also checked in ex vivo microglia isolated from mouse using GW182 immunostaining. CD200R1-associated fluorescence was quantitated in “Image J”. J, representative immunofluorescence stained microglia, (K) scatter plots representing quantitative analysis of GW182 associated immunofluorescence. (n = 4 mice/group; total cells in control group-67 and in arsenic group-60). Scale bar: 5 μm. “n” denotes the number of independent study for in vitro experiments. Bar graphs represent mean ± SEM. “p” denotes the level of significance in comparison to control; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001; ns, nonsignificant.