FIGURE 3.
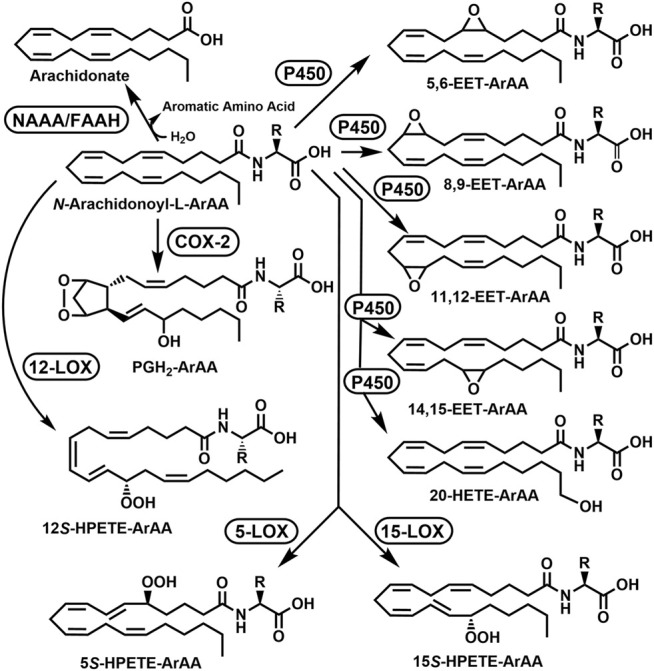
Putative Acyl Group Oxidation and Hydrolytic Degradation of the N-Arachidonoyl Aromatic Amino Acids (ArAAs). The possible metabolites are 5,6-EET-ArAA, N-5,6-epoxyeicosatrienoyl-ArAA; 8,9-EET-ArAA, N-8,9-epoxyeicosatrienoyl-ArAA; 11,12-EET-ArAA, N-11,12-epoxyeicosatrienoyl-ArAA; 14,15-EET-ArAA, N-14,15-epoxyeicosatrienoyl-ArAA; 20-HETE-ArAA, N-20-hydroxyeicosatetraenoyl-ArAA; 5S-HPETE-ArAA, N-(5S-hydroperoxy)-eicosatetraenoyl-ArAA; 12S-HPETE-ArAA, N-(12S-hydroperoxy)-eicosatetraenoyl-ArAA; 15S-HPETE-ArAA, N-(15S-hydroperoxy)-eicosatetraenoyl-ArAA; and PGH2-EA, prostaglandin E2-ArAA. The enzymes would be COX-2, cyclooxygenase-2; FAAH, fatty acid amide hydrolase; 5-LOX, 5-lipoxygenase; 12-LOX, 12-lipoxygenase, 15-LOX, 15-lipoxygenase, NAAA, N-acylethanolamine hydrolyzing acid amidase; and P450, cytochrome P450. The R-group represents the side-groups for the aromatic amino acids shown in Figure 1. This figure is adapted from the modifications of anandamide described in Rouzer and Marnett (2011) and Biringer (2021).
