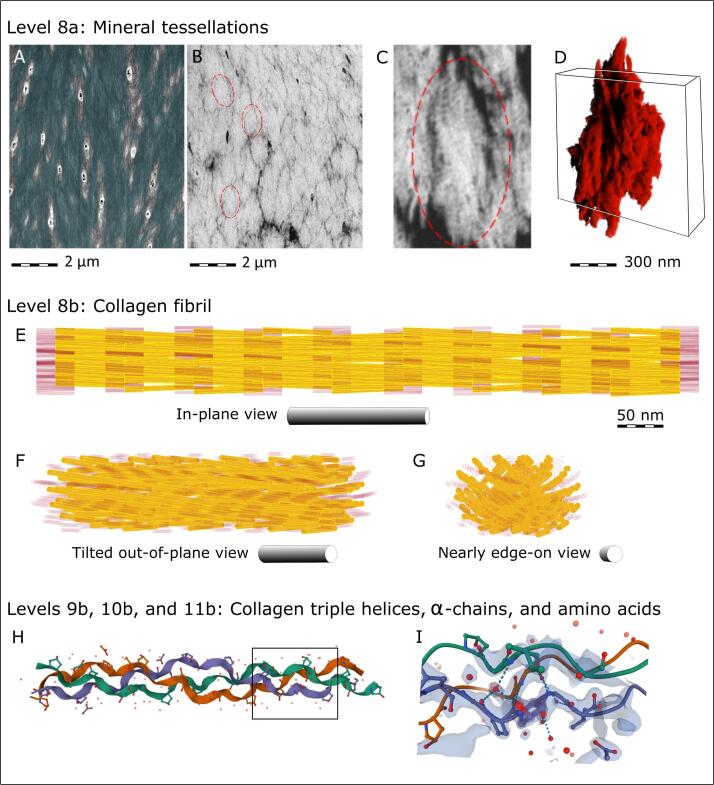
Fig. 5.
Hierarchical Level 8a – prolate ellipsoidal mineral tessellations that populate bundles of ordered collagen fibrils in lamellar bone. (A) 2D view from Fig. 4A and 4C. (B) An orientation- and magnification-matched 2D view of undemineralized bone from Buss et al. (Buss et al., 2020). (C) A further magnified 2D image of a typical mineral aggregate – a “tesselle”. (D) A 3D rendering of a typical single tesselle, as outlined by dashed red ellipses in panels B and C (the tesselle in panel C is one 2D cross-sectional view of its subsequent 3D volume shown in panel D). The rectangular box in panel C roughly corresponds to the complete volume of interest presented in the following Fig. 6. (E) Hierarchical Level 8b showing a supertwisted model of a mineralized collagen fibril with a characteristic stagger of triple helices and a 5° molecular tilt with respect to the fibril axis, reminiscent of the seed arrangement in the sunflower, as suggested by Charvolin and Sadoc (Charvolin and Sadoc, 2011, Charvolin and Sadoc, 2012). Gap regions between collagen triple helices are depicted in transparent red with the hydroxyapatite crystals represented in yellow. (F, G) The same 3D supertwisted collagen model rotated around the vertical axis (“yaw”) to accentuate the resultant spiraling alignment of the gaps and the mineral crystallites that would be confined to the gap zones. Panels H and I show the hierarchical structure of collagen only at the levels of triple helices, α-chains and amino acids (images adapted from the RCSB Protein Data Bank (Bella et al., 1994, Kramer et al., 1998, Sehnal, 2021); https://doi.org/10.2210/pdb1CAG/pdb). The corresponding organizational levels for bone mineral are shown in Fig. 6, Fig. 7.