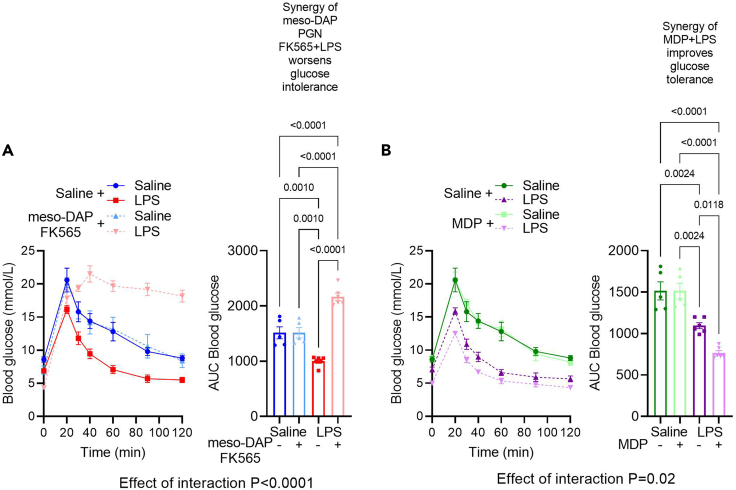
Figure 1.
Postbiotics alter blood glucose control
Exemplar data depicts the expected effect of injection of different types of peptidoglycan (PGN) and lipopolysaccharide (LPS) on blood glucose during a glucose tolerance test in mice. Exemplar data for blood glucose at each time point and area under the curve (AUC) for blood glucose and time following treatment with (A) meso-DAP containing PGN (i.e., FK565) or (B) Muramyl Dipeptide (MDP) each in combination with LPS derived from E. coli during an intraperitoneal (i.p.) glucose tolerance test (2 g/kg D-glucose). Note that NOD1 agonist (i.e., FK565) works in synergy with E. coli LPS and is expected to cause profound glucose intolerance but the NOD2 agonist (i.e., MDP) promotes tolerance and is expected to improve glucose clearance in E. coli LPS injected mice. Data are shown as the mean ± SEM, and the distribution of AUC values was tested using the Shapiro-Wilk test, where normality was confirmed. P values were calculated using two-way analysis of variance (ANOVA) and Tukey multiple comparison test of AUC values. Differences were considered statistically significant at P < 0.05.