Abstract
Diabetic foot ulcers are often unresponsive to conventional therapy and are a leading cause of amputation. Animal studies have shown stem cells and growth factors can accelerate wound healing. Adipose‐derived stem cells are found in fat grafts and mixing them with platelet‐rich plasma (PRP) may improve graft survival. This study aimed to establish the histological changes when diabetic foot ulcers are treated with fat grafts and PRP. A three‐armed RCT was undertaken of 18 diabetic foot ulcer patients: fat grafting; fat grafting with PRP; and routine podiatry care. Biopsies were obtained at week 0, 1, and 4, and underwent quantitative histology/immunohistochemistry (H&E, CD31, and Ki67). Treatment with fat and PRP increased mean microvessel density at 1 week to 1645 (SD 96) microvessels/mm2 (+32%‐45% to other arms, P = .035). PRP appeared to increase vascularity surrounding fat grafts, and histology suggested PRP may enhance fat graft survival. There was no clinical difference between arms. This study demonstrates PRP with fat grafts increased neovascularisation and graft survival in diabetic foot ulcers. The histology was not, however, correlated with wound healing time. Future studies should consider using apoptosis markers and fluorescent labelling to ascertain if enhanced fat graft survival is due to proliferation or reduced apoptosis. Trial registration NCT03085550.
Keywords: adipose‐derived stem cells, diabetic foot ulcers, fat grafting, histology, platelet‐rich plasma
1. INTRODUCTION
Diabetic foot ulcers cost the National Health Service (NHS) over £580 million 1 per annum and are a leading cause of lower‐limb amputations. 2 A UK population‐based cohort study demonstrated that the development of a diabetic foot ulcer is associated with a 5% mortality rate within the first 12 months, increasing to 42% mortality within 5 years, 3 highlighting the gravity of the condition. Currently, treatment consists of podiatry care, vascular optimisation, and prevention of infections. This conventional therapy has inconsistent efficacy with high rates of diabetic foot ulcers transforming into chronic wounds—approximately 20% of patients have an unhealed diabetic foot ulcer at 1 year. 4 Consequently, there is a need for new approaches, including investigation into the use of stem cells 5 and growth factors,6 to promote, accelerate, and restore the body's natural healing process.
Adipose tissue is a source of multipotent stem cells. 7 Adipose‐derived stem cells (ADSC) are capable of self‐renewal, differentiating into cell types involved in tissue repair, and secreting growth factors. 8 ADSC are more abundant than bone marrow derived stem cells 9 and can be easily and repeatedly harvested with minimal donor site morbidity via liposuction. ‘Fat grafting’, where the lipoaspirate containing ADSC and adipocytes is re‐injected, is already widely utilised clinically for soft tissue reconstruction and in aesthetic medicine. 10 Animal studies have shown ADSC administration can improve the rate of cutaneous wound healing by 14% to 47%, 8 , 11 , 12 , 13 , 14 , 15 , 16 including in diabetic animals. 17 , 18 , 19 , 20 , 21 What is more, these studies identified increased angiogenesis 11 , 12 , 13 , 15 , 17 , 18 , 19 , 20 , 21 , 22 , 23 within the first 14 days and increased cell proliferation at 15 days. 21
In order for the ADSCs to participate in wound healing, they must survive the initial grafting procedure. The low survival of all cells within fat grafts is a major issue that limits the use of fat grafting in all settings. 24 A systematic review reported fat graft survival was between 15% and 58% over 4 weeks to 12 months. 25 The methodology to calculate the graft survival rate has lacked scientific rigour, with gross observation 26 and dissection of the graft and weighing 27 , 28 in wide use. In a systematic review of fat grafting 25 it was reported that only one study used 3D objective fat volume measurements through magnetic resonance imaging (MRI), and in this study, the survival rate was just 18%. 29 A lack of oxygen and nutrients from the blood (ischaemia) is considered the main cause of cell loss, and one study has demonstrated adipocytes more than 300 μm from the periphery of the graft become non‐viable within 24 hours. 30
Mixing the fat grafts with platelet‐rich plasma (PRP), an autologous blood product and potent source of growth factors, may enhance graft survival primarily through increased early angiogenesis, which causes faster graft re‐vascularisation. This has been demonstrated in animal studies. 26 , 27 , 28 , 31 PRP may also act through other mechanisms to increase fat graft survival via: (a) inducing the increased proliferation of ADSC, 32 to enable a greater population of ADSC to participate in wound healing; (b) enhancing ADSC differentiation into cells that could directly participate in regeneration and repair (in vitro PRP has been shown to encourage ADSC to differentiate into fibroblasts and keratinocytes); 33 (c) the fibrin component of PRP may enhance fat graft survival through reducing anoikis; 34 or (d) causing further increased secretion of growth factors from ADSC, 35 which would further potentiate early graft re‐vascularisation.
PRP can vary in constitution when manufactured via different commercial methods (over 4‐fold difference in concentration of platelets in one study). 36 The PRP activation process may also vary among PRP studies, which is known to affect the rate of growth factor release, 37 with some studies not activating their PRP at all. The concentration of PRP has also been demonstrated to have differing effects on cells, with higher concentrations (20% in vitro) paradoxically not promoting ADSC proliferation. 38 All of these factors add another layer of complexity to comparing the clinical and histological outcomes from different studies.
The aim of this study was to determine if the local administration of fat grafts with PRP increases wound healing in diabetic foot ulcers at a histological level compared with standard care. Knowledge from this study will establish the feasibility of fat grafting as a novel treatment for these chronic wounds.
2. MATERIALS AND METHODS
A prospective, human randomised single‐centre feasibility study was undertaken. 39 This was a three‐armed study comparing fat grafting, fat grafting with PRP, and routine care for the treatment of diabetic foot ulcers. Of 18 patients enrolled, the first 16 consecutive patients (5 fat grafting only, 6 fat/PRP, and 5 podiatry) underwent wound biopsies. Full details on the inclusion/exclusion criteria, randomisation process, interventions, control, ethics, and trial registration can be found in Supplementary Content S1.
2.1. Histology
Punch biopsies (4 mm) were taken from the wound centre and edge at the initial intervention (day 0), week 1, and week 4. This was performed under local anaesthetic, and biopsies were obtained from all patients regardless of the arm of the study. The samples were stored in 4% formalin in the fridge overnight, and then transferred to 20% sucrose in phosphate‐buffered saline until processing.
Biopsies underwent paraffin infiltration 40 and were moved into tissue micro‐arrays (TMA) in a predetermined randomised pattern. TMAs were tempered (50°C) and manually compacted until well incorporated by eye, 41 sectioned to 4 μm and underwent staining with haematoxylin and eosin (H&E) for microarchitecture, CD31 (Leica Biosystems [reference CD31‐PECAM‐1] 1:1000, ER2 20 minutes, standard polymer protocol) for blood vessels, and Ki67 (Dako antibody [reference M0823] at 4.1 μg/ml, ER1 20 minutes, standard polymer protocol) for cell proliferation using Lecia Biosystems BOND‐MAX Automated IHC/ISH Stainer. Full staining details are in Supplementary Content S2. Slides were scanned and analyses were undertaken in NDP.view v2.5 (Hamamatsu Photonic K.K, 2014) and QuPath. 42
2.2. Epidermal thickness
Average epidermal thickness was calculated by dividing the epidermal cross‐sectional area by the average epidermal length. Measurements were obtained from samples from wound edges. Oblique sections were excluded.
2.3. Microvessel density with CD31 staining
Using a standard technique for quantifying microvessel density, 43 each section was examined at low magnification (x40) to identify areas with the greatest density of stained microvessels. Three non‐overlapping fields of vision (x500 magnification) were selected, and the number of stained microvessels was quantified by manually counting. The area of dermal tissue in the frame was measured (the epidermis was excluded as this is an avascular structure) and the total number of microvessels was divided by the area to give a density.
Any brown staining CD31 positive endothelial cell or endothelial cell cluster that was separate from an adjacent microvessel was counted as a microvessel. 44 A vascular lumen was not required to define a microvessel. 43 Distinct clusters of stained endothelial cells that could be from the same vessel as it moves in and out of the section were also counted separately as individual microvessels. If a vessel had a thick muscular wall or a diameter > 50 μm then these were excluded, as they are unlikely to have been formed recently and are likely preceding mature vessels. See Figure 1A,B.
FIGURE 1.
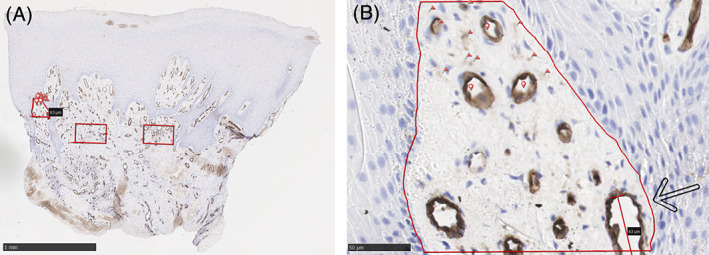
Methods used for quantification of microvessels in dermal tissue from skin biopsies, based on a standard technique 43 for dermal tissue. A, shows three areas are identified visually as having the highest levels of CD31 uptake and therefore highest density of stained microvessels. Three non‐overlapping fields at x500 magnifications (red boxes) are selected. B, shows the left‐most area at higher magnification. The avascular epidermis has not been included in the area. Any brown staining was counted as a microvessel and 10 example microvessels have been marked with a red flag. A lumen was not required to be identified as a microvessel, three example lumens of vessels have been marked with red circular markers for alliteration purposes. Vessels with a thick muscular wall (none shown) or a diameter > 50 μm (marked by black arrow) were excluded from the count. The microvessel count from this particular area was 44, 36, and 49. As this has a > 10% variation it was counted a further two times (41, 45) and a mean taken of all readings (mean = 43). The area was calculated automatically by the software (NPD.view) at 0.0286 mm2. This gave a microvessel density of 1503 microvessels/mm2
Each specimen was triple counted, and a mean calculated. If individual counts differed by more than 10%, two further counts were performed and the mean taken of all five. Microvessel density was measured from edge biopsies preferentially over central wound biopsies, as it was harder to delineate microvessels from background staining sufficiently well. The process was undertaken unblinded by one author.
2.4. Cell proliferation with Ki67 staining
The proportion of Ki67 positive cells 45 was calculated using the positive cell detection program in QuPath. 42 As proliferating cells are located in the basal layer of the epidermis, an area comprising of the dermal‐epidermal junction was selected. Both edge and central wound biopsies containing dermal‐epidermal junction were included. The samples were manually checked to ensure all positive cells were correct and to remove incorrect background staining not related to a positive nucleus. (See Figure S1, Supplementary Content S3) The settings used in QuPath 42 can be found in Supplementary Content S3.
2.5. Statistical analysis
Data from patients receiving the same treatment at the same time points were pooled. Results were analysed by week and intervention arms. Data were analysed using a one‐way analysis of variance (ANOVA) test or Kruskal‐Wallis depending upon distribution. Two‐tailed P values were recorded, with significance set at P < .05. Statistical analysis was performed in SPSS version 26 (IBM, Armonk, New York). In total, 34 samples underwent quantitative analysis of CD31 and Ki67 with five samples being unsuitable due to their orientation and consequently excluded.
3. RESULTS
The clinical results of the RCT can be found in the 2020 paper by Smith et al. 39
3.1. H&E staining
Vertical section of the wound edge and centre can be seen in Figure 2A‐K. Large volumes of organised mature adipocytes are visible in samples at week 1 in the fat/PRP arm, compared with a lower density and less organised adipocytes in the fat graft only arm. When the fat grafts were initially injected into the diabetic ulcers, there were no visible gaps between cells, so background staining between cells may represent where adipocytes have not survived. This suggests greater fat graft survival with the addition of PRP. No adipocytes were visible in the control group who did not undergo fat grafting.
FIGURE 2.
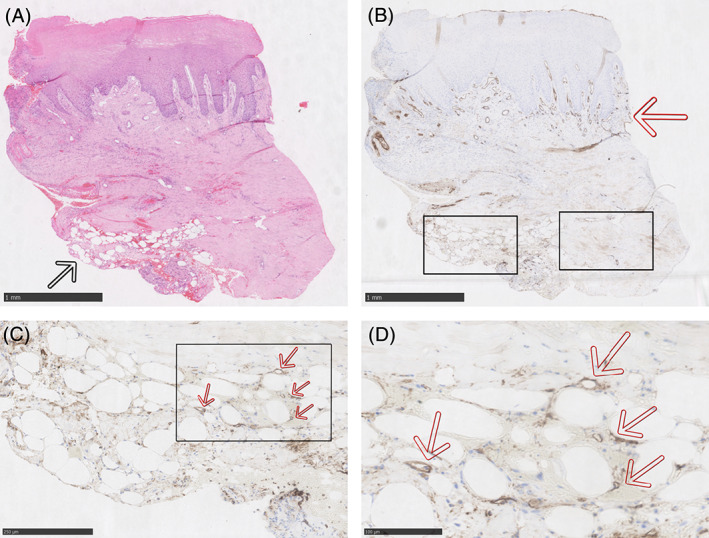
A to F, Histology from an example patient who underwent treatment with fat grafting mixed with PRP. The above samples were all taken at 1‐week post‐intervention from the edge of a diabetic foot ulcer. A, is stained with haematoxylin and eosin (H&E) and mature adipocytes can be seen in the bottom left of the image next to the black arrow. B, is the same section, from the same patient at the same time point stained with CD31 (an endothelial marker for blood vessels). The blood vessels in the reticular dermis stain brown/black and can be visualised in line with the red arrow. The epidermis is avascular. There also appears to be increased CD31 uptake around the adipocytes in the bottom left where the sample has been treated with fat grafting and PRP. C, is the lower left box from B in higher magnification, and D, is the box in C magnified. Individual microvessels can be seen, which have formed around the adipocytes. There is also increased uptake of CD31 generally amongst the adipocytes suggesting there are many more microvessels that are too small to be fully appreciated. Several microvessels as examples have been labelled with red arrows; however, similar small brown lumens can be seen around every adipocyte. E, in contrast is the lower right box from B and shows an area where there was not grafted fat/PRP. There is a paucity of microvessels in this field of view. Instead there are two large mature vessels that can be seen transected in the box (marked with red arrows). There is less uptake of CD31 throughout also, again suggesting a lack of microvessels. F, is stained with Ki67 (cellular proliferation. Uptake of Ki67 can be seen primarily at the dermal‐epidermal junction and is occurring in the basal layer of the epidermis. Otherwise there is a similar proportion of Ki67 positive staining cells throughout the sample and there is no association with the grafted fat/PRP in the bottom left of the slide. G and H are H&E staining of a central wound biopsy in a patient in the fat grafting mixed with PRP study arm, 1 week following treatment. The adipocytes from the fat graft are shown near the back arrow. A large number of adipocytes have survived and they appear well organised. H, is the area by the back arrow at higher magnification where the organisation be more clearly appreciated. I to K, are H&E staining of a central wound biopsy in a patient in the fat grafting only study arm, 1 week following treatment. Mature rounded adipocytes are again visible at the peripheries of the central biopsy (black and blue arrows). In comparison to the fat/PRP sample above, the adipocytes are less densely packed, less numerous, and less well organised. This suggests that survival has not been achieved by as many grafted cells. J and K are higher magnification by the black and blue arrows, respectively. At higher magnification the lower density of adipocytes and lower level of organisation can be more easily appreciated. More pink staining tissue can be seen between cells in this slide compared with the fat/PRP sample
Epithelial thickness was not affected by any of the intervention arms and there was no significant difference between any group at any time point (P = .115), see Figure 3.
FIGURE 3.
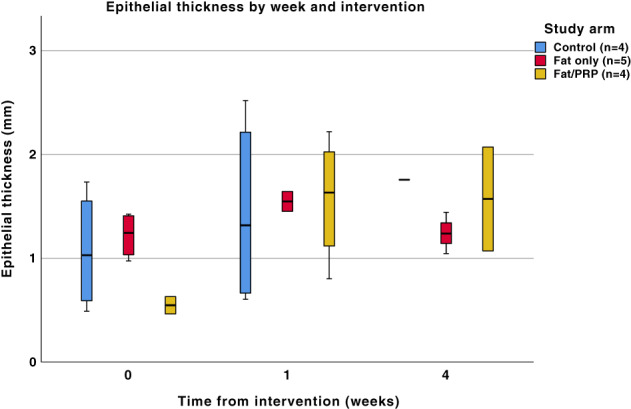
A boxplot of epithelial thickness (mm) from H&E staining at three time points in the different study arms following the various interventions. There was no statistically significant difference between any of the groups at any time point (P = .115). Not all samples could be included at every time point due to some being oblique sections and therefore unsuitable for this analysis
3.2. CD31 staining
A statistically significant difference in the density of microvessels was seen at 1‐week post‐intervention (P = .035) in the fat/PRP group. There was no difference between the study groups at the time of intervention (week 0, P = .797) and 4 weeks post‐intervention (P = .152), see Figure 4. When the results were analysed by intervention across the three time points, similar results were found with only the fat/PRP group trending towards significance (P = .071). Control and fat groups were not statistically significant between weeks (P = .926 and P = .103, respectively). The epidermis contained no CD31 staining blood vessels.
FIGURE 4.
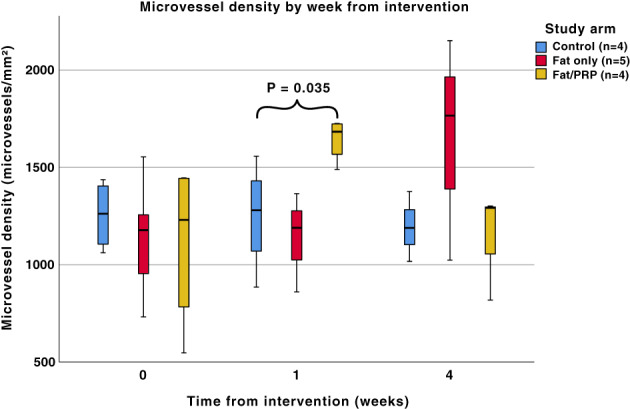
A boxplot of microvessel density (vessels per mm2) at time points in the different study arms following the various interventions. A statistically significant difference was seen at 1‐week post‐intervention between the three treatment groups (P = .035). No significant difference in microvessel density was observed between the groups at the time of intervention (week 0, P = .797) and 4 weeks post‐intervention (P = .152). When analysed by intervention across the three time points, fat/PRP trended towards statistical significance (P = .071). The remaining P values for fat only were .103 and for controls were .926
3.3. Ki67 staining
Ki67 positive cells were located in the basal layer of the epidermis throughout all of the samples. There was large variation within individual samples, with some areas displaying no Ki67 positive cells are other areas with a high proportion of positive cells.
A significant difference was found in the control group (P = .047) across the three time points, between weeks 1 and 4. There was no difference in either of the intervention groups across the different time points (P = .338 for fat, P = .304 for fat/PRP). When analysed by week, there was also no difference in Ki67 positive cells (week 0 P = .221, week 1 P = .231, week 4 P = .05), see Figure 5.
FIGURE 5.
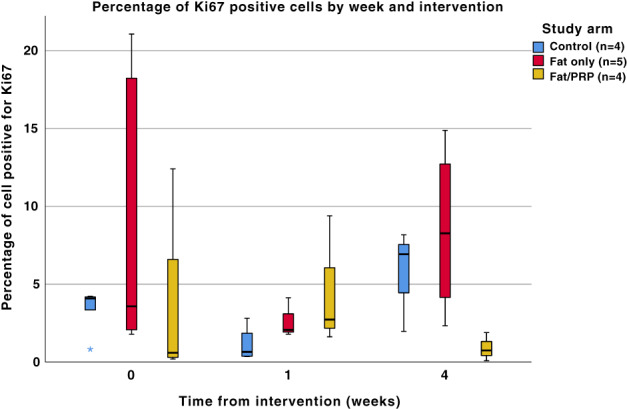
The proportion of cells staining positive for Ki67 by week and intervention. There was a statistically significant change in the control group over time (P = .047). There was no difference in either of the intervention groups across the different time points (P = .338 for fat only, P = .304 for fat/PRP). When analysed by week there was also no difference (week 0 P = .221, week 1 P = .231, week 4 P = .05)
4. DISCUSSION
This study found the application of fat with PRP to diabetic foot ulcers caused increased angiogenesis at 1 week, which was statistically significant. The addition of PRP also increased the fat graft survival, as demonstrated by increased density of adipocytes seen in central wound biopsies. Neither fat grafts nor fat/PRP had any effect on cell proliferation or epithelial thickness. This is the first clinical study to provide evidence that autologous fat grafts and PRP can augment wound healing of diabetic foot ulcers at a histological level.
A major factor that hinders the ability of diabetic foot ulcers to heal spontaneously is reduced angiogenesis. 46 Previous animal studies have shown that ADSC 8 , 11 , 12 , 13 , 14 , 15 , 17 , 18 , 19 , 20 , 21 and PRP 26 , 27 , 28 , 31 amplify angiogenesis. Animal studies using models of impaired wound healing often found a greater effect with ADSC/fat grafts and PRP than in healthy subjects. 17 , 18 , 19 , 20 , 21 Our findings of increased angiogenesis in human diabetic ulcers following treatment with fat grafts and PRP are encouraging. Previously it was not known how diabetes would reduce the wound healing capacity of the autologous fat grafts and ADSC. Studies have reported that diabetes reduces ADSC's proliferation, differentiation, and proangiogenic properties47, 48, 49 which were hypothesised to attenuate their therapeutic potential in wound healing. The composition of PRP is also affected by ageing, diabetes, and anti‐platelet medications, which reduce the concentration of some growth factors. 50 This study demonstrates that despite the reduced potency of ADSC and PRP in diabetic patients, these two autologous materials together positively affect the histology of diabetic ulcers.
Cell proliferation in the basal layer of the epidermis was not affected by our interventions at any time point. Statistically significant changes were observed in in the control group, where a reduction was seen at week 4 compared with week 0 and week 1. The clinical significance of this is unclear and this finding may be a type 1 error. Previously one animal study reported that ADSC increased Ki67 and cell proliferation 21 while another reported no change. 11 Large differences in the proportion of positive cells by a factor of >250 times were observed in our study (ranging from 0.08% to 21%), however wide variation in a single sample was also observed. One hypothesis explaining these conflicting results is that cellular proliferation may be affected in specific parts of the wound, or tissue very close to ADSC or PRP. No differences were also seen in the epithelial thickness between any of the study arms at any time point.
The fundamental issue of low‐fat graft survival hinders its clinical use in all settings. 24 This study demonstrated increased graft survival with the addition of PRP. This finding was based on the observation of increased density of adipocytes in biopsies, which may be limited by the small areas of tissue observed containing fat grafts. While assessment using 3D imaging like micro or nano computerised topography may be technically possible, it is important to remember that non‐viable adipocytes still have a volume, so these techniques also have their drawbacks. Increased adipocyte viability with the addition of activated PRP has been identified in many animal studies, 16 , 26 , 27 , 28 , 31 , 51 hypothesised to be due to the uncoordinated release of growth factors from the platelets causing increased neo‐angiogenesis within the initial 24 hours. Our study confirms this in diabetic individuals in addition. We identified increased microscopic organisation of fat grafts following addition of PRP, which has also been similarly noted in previous animal studies. 51 , 52 The fibrin component of PRP may be acting as a scaffold for the adipocytes.
In the large body of animal studies on the topic, 8 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 21 the histological changes identified in this study were correlated with the clinical acceleration of wound healing; it is possible that the same is true in humans. It is worth noting the quality of animal studies is inconsistent, as many have small sample sizes, 53 , 54 inconsistent methodologies, and little or no blinding. Most animal studies used rodents, which unlike humans heal 90% of cutaneous wounds through contraction; 55 the host animals used to emulate diabetes only mimic certain aspects of the disease, and despite severe hyperglycaemia, they share few other clinical findings of the syndrome of diabetes mellitus; the ADSC often came from healthy donors rather than autologous ‘diabetic’ individuals; animal studies used ADSC, which has been cultured through 3 to 5 passages, whereas ADSC only make up at most up to 1% 9 of fat grafts. Many of these factors cannot be overcome with further animal studies, hence the need for clinical studies such as this to ascertain the usefulness.
4.1. Limitations
The approach used to measure fat graft survival (visual comparison of the density of adipocytes) had limitations compared with other approaches, such as apoptosis markers or florescent labelling. Future use of similar techniques may additionally answer key questions such as whether the cells in the fat grafts are replicating to make up for lost cells, or a higher proportion surviving the grafting process. Increased microvessel density was also observed at 1 week; however, it is not clear which cell type is responsible for this. VEGF staining may add weight to the observations such as the increased microvessels surrounding fat grafts. Finally, the study size in this feasibility trial was small, and future work should work towards an appropriately powered study population size following a power calculation.
5. CONCLUSIONS
Diabetic foot ulcers are a significant problem, which urgently require novel, efficacious, evidence‐based treatments. Preceding animal studies have shown PRP and ADSC can augment wound healing at a histological and clinical level, however, the quality of these studies is inconsistent. Our clinical study found the application of fat grafts and PRP to diabetic foot ulcers increased angiogenesis, and the histology suggests that fat graft survival was enhanced. These histological improvements were not correlated with clinical improvements. Nonetheless, the results are encouraging, and future work should consider further techniques to establish the cause of the enhanced fat graft survival, and subsequently promote the conception of novel treatments for diabetic foot ulcers.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Supporting information
Appendix S1. Content 1.
Appendix S2. Content 2 and 3.
Figure S1 Supporting Information.
ACKNOWLEDGEMENTS
We would like to thank the patients of the Royal Free who were involved and the podiatry team for their help and support with this project. We would also like to thank Al Manning for his advice on statistics and Flora Campbell for her assistance with histology interpretation.
Nolan GS, Smith OJ, Heavey S, Jell G, Mosahebi A. Histological analysis of fat grafting with platelet‐rich plasma for diabetic foot ulcers—A randomised controlled trial. Int Wound J. 2022;19(2):389–398. 10.1111/iwj.13640
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Kerr M. Diabetic foot care in England: an economic study Diabetes UK; 2017.
- 2. Pecoraro RE, Reiber GE, Burgess EM. Pathways to diabetic limb amputation. Basis for prevention. Diabetes Care. 1990;13(5):513‐521. [DOI] [PubMed] [Google Scholar]
- 3. Walsh JW, Hoffstad OJ, Sullivan MO, Margolis DJ. Association of diabetic foot ulcer and death in a population‐based cohort from the United Kingdom. Diabet Med. 2016;33(11):1493‐1498. 10.1111/dme.13054. [DOI] [PubMed] [Google Scholar]
- 4. Prompers L, Schaper N, Apelqvist J, et al. Prediction of outcome in individuals with diabetic foot ulcers: focus on the differences between individuals with and without peripheral arterial disease. The EURODIALE study. Diabetologia. 2008;51(5):747‐755. 10.1007/s00125-008-0940-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25(10):2648‐2659. 10.1634/stemcells.2007-0226. [DOI] [PubMed] [Google Scholar]
- 6. Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic‐Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16(5):585‐601. 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- 7. Gimble J, Guilak F. Adipose‐derived adult stem cells: isolation, characterization, and differentiation potential. Cytotherapy. 2003;5(5):362‐369. 10.1080/14653240310003026. [DOI] [PubMed] [Google Scholar]
- 8. Kim WS, Park BS, Sung JH, et al. Wound healing effect of adipose‐derived stem cells: a critical role of secretory factors on human dermal fibroblasts. J Dermatol Sci. 2007;48(1):15‐24. 10.1016/j.jdermsci.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 9. Fraser JK, Wulur I, Alfonso Z, Hedrick MH. Fat tissue: an underappreciated source of stem cells for biotechnology. Trends Biotechnol. 2006;24(4):150‐154. 10.1016/j.tibtech.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 10. Simonacci F, Bertozzi N, Grieco MP, Grignaffini E, Raposio E. Procedure, applications, and outcomes of autologous fat grafting. Ann Med Surg (Lond). 2017;20:49‐60. 10.1016/j.amsu.2017.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Altman AM, Yan Y, Matthias N, et al. IFATS collection: human adipose‐derived stem cells seeded on a silk fibroin‐chitosan scaffold enhance wound repair in a murine soft tissue injury model. Stem Cells. 2009;27(1):250‐258. 10.1634/stemcells.2008-0178. [DOI] [PubMed] [Google Scholar]
- 12. Ebrahimian TG, Pouzoulet F, Squiban C, et al. Cell therapy based on adipose tissue‐derived stromal cells promotes physiological and pathological wound healing. Arterioscler Thromb Vasc Biol. 2009;29(4):503‐510. 10.1161/ATVBAHA.108.178962. [DOI] [PubMed] [Google Scholar]
- 13. Huang SP, Hsu CC, Chang SC, et al. Adipose‐derived stem cells seeded on acellular dermal matrix grafts enhance wound healing in a murine model of a full‐thickness defect. Ann Plast Surg. 2012;69(6):656‐662. 10.1097/SAP.0b013e318273f909. [DOI] [PubMed] [Google Scholar]
- 14. Lee SH, Lee JH, Cho KH. Effects of human adipose‐derived stem cells on cutaneous wound healing in nude mice. Ann Dermatol. 2011;23(2):150‐155. 10.5021/ad.2011.23.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nambu M, Ishihara M, Nakamura S, et al. Enhanced healing of mitomycin C‐treated wounds in rats using inbred adipose tissue‐derived stromal cells within an atelocollagen matrix. Wound Repair Regen. 2007;15(4):505‐510. 10.1111/j.1524-475X.2007.00258.x. [DOI] [PubMed] [Google Scholar]
- 16. Nolan GS, Smith OJ, Jell G, Mosahebi A. Fat grafting and platelet‐rich plasma in wound healing: a review of histology from animal studies. Adipocytes. 2021;10(1):80‐90. 10.1080/21623945.2021.1876374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kato Y, Iwata T, Morikawa S, Yamato M, Okano T, Uchigata Y. Allogeneic transplantation of an adipose‐derived stem cell sheet combined with artificial skin accelerates wound healing in a rat wound model of type 2 diabetes and obesity. Diabetes. 2015;64(8):2723‐2734. 10.2337/db14-1133. [DOI] [PubMed] [Google Scholar]
- 18. Nambu M, Kishimoto S, Nakamura S, et al. Accelerated wound healing in healing‐impaired db/db mice by autologous adipose tissue‐derived stromal cells combined with atelocollagen matrix. Ann Plast Surg. 2009;62(3):317‐321. 10.1097/SAP.0b013e31817f01b6. [DOI] [PubMed] [Google Scholar]
- 19. Nambu M, Ishihara M, Kishimoto S, et al. Stimulatory effect of autologous adipose tissue‐derived stromal cells in an Atelocollagen matrix on wound healing in diabetic db/db mice. J Tissue Eng. 2011;2011:158105. 10.4061/2011/158105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nie C, Yang D, Xu J, Si Z, Jin X, Zhang J. Locally administered adipose‐derived stem cells accelerate wound healing through differentiation and vasculogenesis. Cell Transplant. 2011;20(2):205‐216. 10.3727/096368910X520065. [DOI] [PubMed] [Google Scholar]
- 21. Shi R, Jin Y, Cao C, et al. Localization of human adipose‐derived stem cells and their effect in repair of diabetic foot ulcers in rats. Stem Cell Res Ther. 2016;7(1):155. 10.1186/s13287-016-0412-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Uysal AC, Mizuno H, Tobita M, Ogawa R, Hyakusoku H. The effect of adipose‐derived stem cells on ischemia‐reperfusion injury: immunohistochemical and ultrastructural evaluation. Plast Reconstr Surg. 2009;124(3):804‐815. 10.1097/PRS.0b013e3181b17bb4. [DOI] [PubMed] [Google Scholar]
- 23. Albayati A, Ozkan B, Eyuboglu AA, Uysal AC, Ertas NM, Haberal M. Nonmelanoma skin cancers in solid‐organ transplant recipients: a single center experience. Exp Clin Transplant. 2018;16(Supplement 1):95‐100. [DOI] [PubMed] [Google Scholar]
- 24. Nolan GS, Smith OJ, Mosahebi A. Enhancing fat graft survival with autologous growth factors: platelet‐rich fibrin (PRF) vs platelet‐rich plasma (PRP). Aesthet Surg J. 2021;41(5):NP241. 10.1093/asj/sjaa274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yu NZ, Huang JZ, Zhang H, et al. A systemic review of autologous fat grafting survival rate and related severe complications. Chin Med J. 2015;128(9):1245‐1251. 10.4103/0366-6999.156142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nakamura S, Ishihara M, Takikawa M, et al. Platelet‐rich plasma (PRP) promotes survival of fat‐grafts in rats. Ann Plast Surg. 2010;65(1):101‐106. 10.1097/SAP.0b013e3181b0273c. [DOI] [PubMed] [Google Scholar]
- 27. Pires Fraga MF, Nishio RT, Ishikawa RS, Perin LF, Helene A, Malheiros CA. Increased survival of free fat grafts with platelet‐rich plasma in rabbits. J Plast Reconstr Aesthet Surg. 2010;63(12):e818‐e822. 10.1016/j.bjps.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 28. Oh DS, Cheon YW, Jeon YR, Lew DH. Activated platelet‐rich plasma improves fat graft survival in nude mice: a pilot study. Dermatol Surg. 2011;37(5):619‐625. 10.1111/j.1524-4725.2011.01953.x. [DOI] [PubMed] [Google Scholar]
- 29. Kruschewsky LS, de Mello‐Filho FV, dos Santos AC, Rosen CA. Autologous fat graft absorption in unilateral paralyzed canine vocal folds. Laryngoscope. 2007;117(1):96‐100. 10.1097/01.mlg.0000245013.98844.30. [DOI] [PubMed] [Google Scholar]
- 30. Eto H, Kato H, Suga H, et al. The fate of adipocytes after nonvascularized fat grafting: evidence of early death and replacement of adipocytes. Plast Reconstr Surg. 2012;129(5):1081‐1092. 10.1097/PRS.0b013e31824a2b19. [DOI] [PubMed] [Google Scholar]
- 31. Atashi F, André‐Lévigne D, Colin DJ, Germain S, Pittet‐Cuénod B, Modarressi A. Does non‐activated platelet‐rich plasma (PRP) enhance fat graft outcome? An assessment with 3D CT‐scan in mice. J Plast Reconstr Aesthet Surg. 2019;72(4):669‐675. 10.1016/j.bjps.2018.12.039. [DOI] [PubMed] [Google Scholar]
- 32. Liao HT, Marra KG, Rubin JP. Application of platelet‐rich plasma and platelet‐rich fibrin in fat grafting: basic science and literature review. Tissue Eng Part B Rev. 2014;20(4):267‐276. 10.1089/ten.TEB.2013.0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stessuk T, Puzzi MB, Chaim EA, et al. Platelet‐rich plasma (PRP) and adipose‐derived mesenchymal stem cells: stimulatory effects on proliferation and migration of fibroblasts and keratinocytes in vitro. Arch Dermatol Res. 2016;308(7):511‐520. 10.1007/s00403-016-1676-1. [DOI] [PubMed] [Google Scholar]
- 34. Aoyagi Y, Kuroda M, Asada S, et al. Fibrin glue is a candidate scaffold for long‐term therapeutic protein expression in spontaneously differentiated adipocytes in vitro. Exp Cell Res. 2012;318(1):8‐15. 10.1016/j.yexcr.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 35. Siegel KR, Clevenger TN, Clegg DO, Proctor DA, Proctor CS. Adipose stem cells incorporated in fibrin clot modulate expression of growth factors. Arthroscopy. 2018;34(2):581‐591. 10.1016/j.arthro.2017.08.250. [DOI] [PubMed] [Google Scholar]
- 36. Fitzpatrick J, Bulsara MK, McCrory PR, Richardson MD, Zheng MH. Analysis of platelet‐rich plasma extraction: variations in platelet and blood components between 4 common commercial kits. Orthop J Sports Med. 2017;5(1):2325967116675272. 10.1177/2325967116675272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cavallo C, Roffi A, Grigolo B, et al. Platelet‐rich plasma: the choice of activation method affects the release of bioactive molecules. Biomed Res Int. 2016;2016:6591717. 10.1155/2016/6591717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kakudo N, Minakata T, Mitsui T, Kushida S, Notodihardjo FZ, Kusumoto K. Proliferation‐promoting effect of platelet‐rich plasma on human adipose‐derived stem cells and human dermal fibroblasts. Plast Reconstr Surg. 2008;122(5):1352‐1360. 10.1097/PRS.0b013e3181882046. [DOI] [PubMed] [Google Scholar]
- 39. Smith OJ, Leigh R, Kanapathy M, et al. Fat grafting and platelet‐rich plasma for the treatment of diabetic foot ulcers: a feasibility‐randomised controlled trial. Int Wound J 2020;17(6):1578‐1594. 10.1111/iwj.13433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Canene‐Adams K. Preparation of formalin‐fixed paraffin‐embedded tissue for immunohistochemistry. Methods Enzymol. 2013;533:225‐233. 10.1016/B978-0-12-420067-8.00015-5. [DOI] [PubMed] [Google Scholar]
- 41. Chang H, Peluso D, Hussain S, Shipitsin M, Blume‐Jensen P. Optimizing tissue microarray construction procedure to improve quality of sections. J Histotechnol 2014. 37(3):95–100. doi: 10.1179/2046023614Y.0000000046 [DOI] [Google Scholar]
- 42. Bankhead P, Loughrey MB, Fernández JA, et al. QuPath: open source software for digital pathology image analysis. Sci Rep. 2017;7(1):16878. 10.1038/s41598-017-17204-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Saravanamuthu J, Reid WM, George DS, et al. The role of angiogenesis in vulvar cancer, vulvar intraepithelial neoplasia, and vulvar lichen sclerosus as determined by microvessel density analysis. Gynecol Oncol. 2003;89(2):251‐258. 10.1016/s0090-8258(03)00055-6. [DOI] [PubMed] [Google Scholar]
- 44. Weidner N. Current pathologic methods for measuring intratumoral microvessel density within breast carcinoma and other solid tumors. Breast Cancer Res Treat. 1995;36(2):169‐180. 10.1007/bf00666038. [DOI] [PubMed] [Google Scholar]
- 45. Tang LH, Gonen M, Hedvat C, Modlin IM, Klimstra DS. Objective quantification of the Ki67 proliferative index in neuroendocrine tumors of the gastroenteropancreatic system: a comparison of digital image analysis with manual methods. Am J Surg Pathol. 2012;36(12):1761‐1770. 10.1097/PAS.0b013e318263207c. [DOI] [PubMed] [Google Scholar]
- 46. Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366(9498):1736‐1743. 10.1016/S0140-6736(05)67700-8. [DOI] [PubMed] [Google Scholar]
- 47. Kim HK, Kim YJ, Kim JT, et al. Alterations in the proangiogenic functions of adipose tissue‐derived stromal cells isolated from diabetic rats. Stem Cells Dev. 2008;17(4):669‐680. 10.1089/scd.2007.0141. [DOI] [PubMed] [Google Scholar]
- 48. Cianfarani F, Toietta G, Di Rocco G, Cesareo E, Zambruno G, Odorisio T. Diabetes impairs adipose tissue‐derived stem cell function and efficiency in promoting wound healing. Wound Repair Regen. 2013;21(4):545‐553. 10.1111/wrr.12051. [DOI] [PubMed] [Google Scholar]
- 49. Varghese J, Griffin M, Mosahebi A, Butler P. Systematic review of patient factors affecting adipose stem cell viability and function: implications for regenerative therapy. Stem Cell Res Ther. 2017;8(1):45. 10.1186/s13287-017-0483-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tian J, Lei XX, Xuan L, Tang JB, Cheng B. The effects of aging, diabetes mellitus, and antiplatelet drugs on growth factors and anti‐aging proteins in platelet‐rich plasma. Platelets. 2019;30(6):773‐792. 10.1080/09537104.2018.1514110. [DOI] [PubMed] [Google Scholar]
- 51. Hersant B, Bouhassira J, SidAhmed‐Mezi M, et al. Should platelet‐rich plasma be activated in fat grafts? An animal study. J Plast Reconstr Aesthet Surg. 2018;71(5):681‐690. 10.1016/j.bjps.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 52. Rodríguez‐Flores J, Palomar‐Gallego MA, Enguita‐Valls AB, Rodríguez‐Peralto JL, Torres J. Influence of platelet‐rich plasma on the histologic characteristics of the autologous fat graft to the upper lip of rabbits. Aesthet Plast Surg. 2011;35(4):480‐486. 10.1007/s00266-010-9640-5. [DOI] [PubMed] [Google Scholar]
- 53. Carter CA, Jolly DG, Worden CE, Hendren DG, Kane CJ. Platelet‐rich plasma gel promotes differentiation and regeneration during equine wound healing. Exp Mol Pathol. 2003;74(3):244‐255. [DOI] [PubMed] [Google Scholar]
- 54. DeRossi R, Coelho AC, Mello GS, et al. Effects of platelet‐rich plasma gel on skin healing in surgical wound in horses. Acta Cir Bras 2009. 24(4):276–81. [DOI] [PubMed] [Google Scholar]
- 55. Greenhalgh DG. Models of wound healing. J Burn Care Rehabil. 2005;26(4):293‐305. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Content 1.
Appendix S2. Content 2 and 3.
Figure S1 Supporting Information.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


