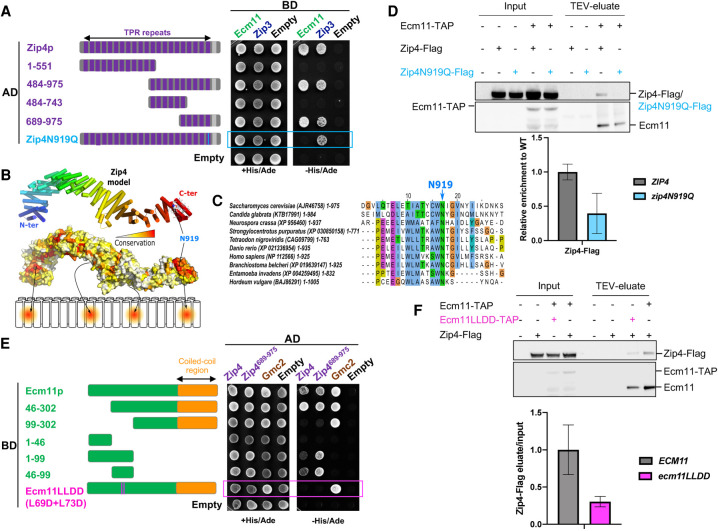
Figure 2.
Zip4 specifically interacts with Ecm11. (A) Delineation of the Ecm11-interacting domain in Zip4 by two-hybrid assays. The indicated fragments of Zip4 were fused to GAL4-AD and tested in combination with a GAL4-BD-Ecm11 or GAL4-BD-Zip3 fusion. The blue frame indicates the absence of interaction between Zip4N919Q and Ecm11. (B) 3D model of Zip4 TPR revealing four conserved surface patches. The degree of conservation is shown. (C) Alignment of the Zip4 C-terminal TPR domain. (D) Coimmunoprecipitation between Zip4-Flag, Zip4N919Q-Flag, and Ecm11-TAP from meiotic cells at 5 h in meiosis, analyzed by Western blot. Levels of Zip4-Flag and Zip4N919Q-Flag were quantified relative to the input and normalized by Ecm11-TAP levels. Values are the mean ± SD of two independent experiments. (E) Same assay as in A. Ecm11 domains were fused to GAL4-AD and tested in combination with GAL4-BD-Zip4 or GAL4-BD-Zip4-689–971. The pink frame indicates the loss of interaction between Ecm11LLDD and Zip4. (F) Coimmunoprecipitation of Zip4-Flag with Ecm11-TAP or with Ecm11LLDD-TAP from meiotic cells at 4 h in meiosis, analyzed by Western blot. Levels of Zip4-Flag coimmunoprecipitated with Ecm11-TAP or with Ecm11LLDD-TAP were quantified relative to the input and normalized by Ecm11-TAP or Ecm11LLDD-TAP levels. Values are the mean ± SD of two independent experiments.