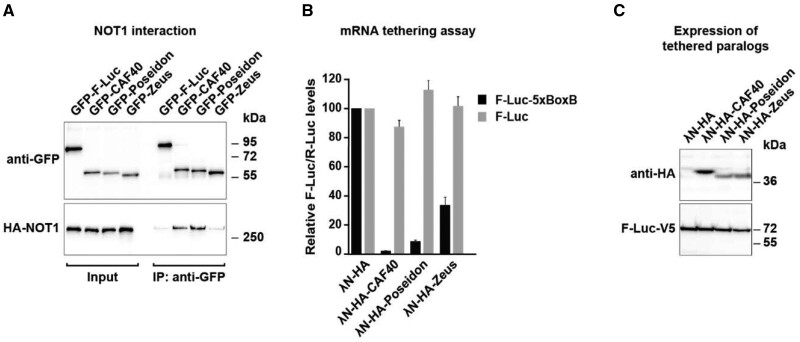
Fig. 5.
CAF40, Poseidon, and Zeus protein interaction with the CCR4–NOT complex. (A) Co-immunoprecipitation assay showing the interaction of CAF40 paralogs with NOT1 in Dm S2 cells. Co-immunoprecipitation was conducted in the presence of RNase A to exclude RNA-mediated interactions. Cell lysates expressing GFP-tagged CAF40, Poseidon and Zeus, and HA-tagged NOT1. GFP-F-Luc served as a negative control. Input samples consist of 3% for the GFP-tagged proteins and 1% for the HA-tagged proteins, and immunoprecipitated samples of 10% for the GFP-tagged proteins and 30% for the HA-tagged proteins. Protein size markers are shown on the right in each panel. (B) Tethering assay using λN-HA-tagged CAF40, Poseidon and Zeus and the F-Luc-5BoxB reporter in Dm S2 cells (black bars). A plasmid expressing R-Luc served as a transfection control, and an F-Luc reporter lacking the 5BoxB binding sites for λN- was used as control (gray bars). F-Luc activity levels were normalized to those of the R-Luc control and set to 100% in cells expressing the λN-HA peptide alone. Error bars indicate SD of five replicates. CAF40 and Poseidon exhibit similar abilities of repressing the luciferase reporter (black bar) compared with the control (gray bar). Zeus exhibits lower, though still significant, repression ability. (C) Western-blot analysis showing the equivalent expression of the λN-HA-tagged proteins used in the tethering assays shown in (B). Protein size markers (kDa) are shown on the right of the panel.