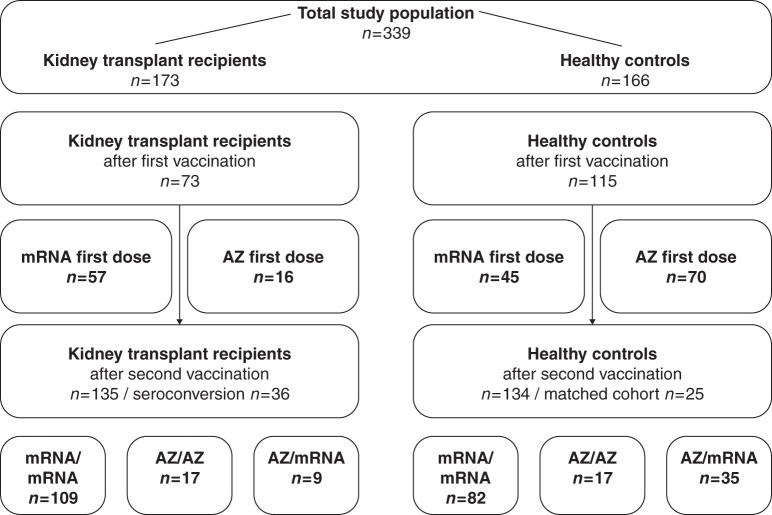
Figure 1.
Study population to determine humoral immune responses to different severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) two-dose vaccination regimens in kidney transplant recipients and healthy controls. In total, 339 participants were included in this study. Humoral response after vaccination was assessed in 173 kidney transplant recipients and 166 healthy controls. Analysis was done for 73 kidney transplant recipients and 115 healthy controls after first vaccination. Kidney transplant recipients and healthy controls received either an mRNA vaccine (n=57 and n=45, respectively) or an adenoviral vector–based vaccine (AZ; n=16 and n=70, respectively) as the first vaccine dose. Humoral response after the two-dose vaccination was assessed in 135 kidney transplant recipients and 134 healthy controls. Kidney transplant recipients received homologous mRNA/mRNA (n=109), homologous AZ/AZ (n=17), or heterologous AZ/mRNA two-dose vaccination (n=9). Healthy controls received homologous mRNA/mRNA (n=82), homologous AZ/AZ (n=17), or heterologous AZ/mRNA two-dose vaccination (n=35). For analysis of antibodies against various SARS-CoV-2 target epitopes and for analysis of neutralization against different variants of concern, kidney transplant recipients were compared with 25 healthy controls matched for type of vaccine, age, and sex.