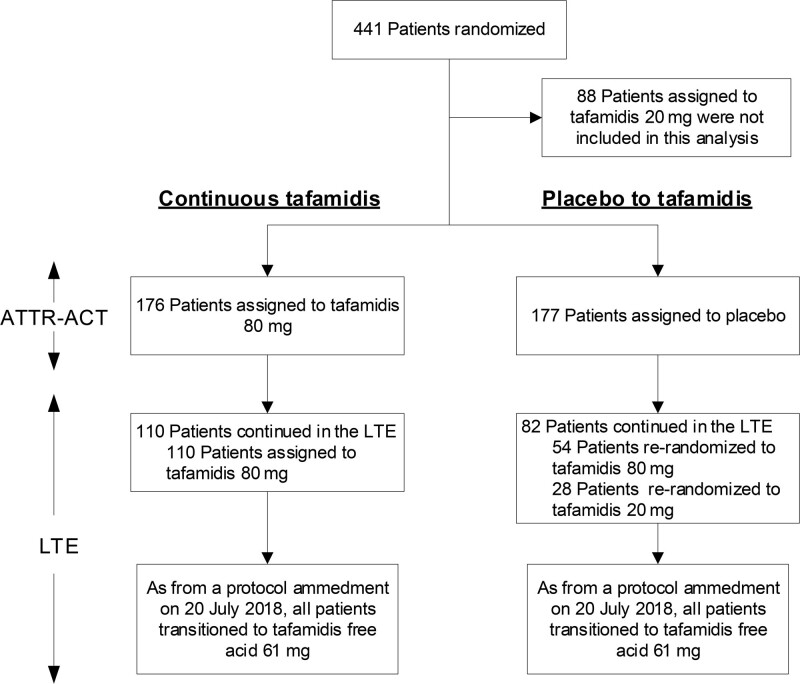
Figure 1.
Patients in ATTR-ACT (Tafamidis in Transthyretin Cardiomyopathy Clinical Trial) and the long-term extension (LTE) in this analysis. Showing the treatment groups (continuous tafamidis and placebo to tafamidis) in ATTR-ACT and the LTE included in this analysis. After 30 months of treatment in ATTR-ACT, patients treated with tafamidis 80 mg could continue in the LTE on the same dose of tafamidis, and patients treated with placebo were randomized (2:1) to tafamidis 80 or 20 mg in the LTE. A total of 110 patients treated with tafamidis 80 mg continued in the LTE. A total of 82 placebo-treated patients continued in the LTE. Following a protocol amendment on July 20, 2018, all patients transitioned to tafamidis free acid 61 mg (bioequivalent to tafamidis 80 mg). ATTR-ACT also included a treatment arm for tafamidis 20 mg, which was not included in this analysis.