Fig. 6.
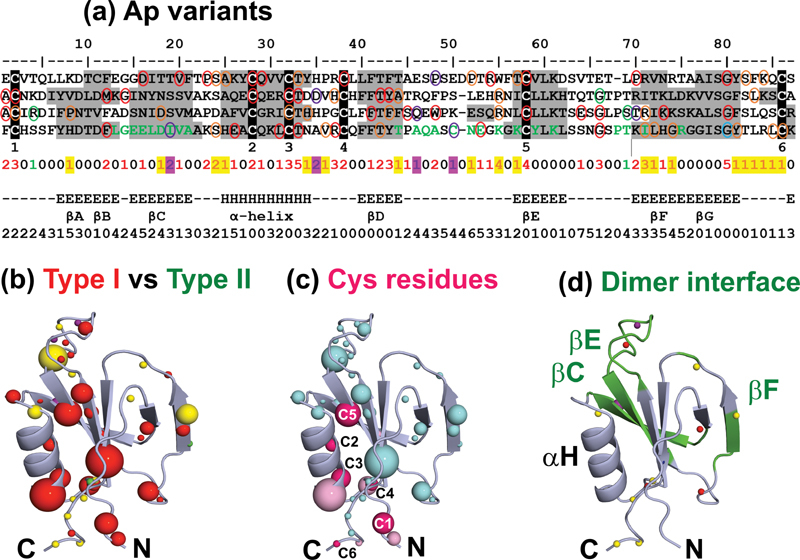
Mutational residue frequency in terms of a consensus Ap domain. A consensus Ap domain of length 87 residues shows the distribution of 90 Ap variants (missense and polymorphisms) in the four Ap1-Ap4 domains merged together. (a) The four Ap domain sequences are aligned to form a consensus, with the averaged secondary structure (Sec) and accessibility (Acc) of each residue listed directly below. The ‘Tot’ row illustrates the total number of variants found at each residue in the consensus. The six conserved Cys residues in each domain are highlighted in black and numbered 1–6 underneath the alignment. The Cys-Cys bridges occur at Ap1 (Cys20-Cys103, Cys46-Cys76, Cys50-Cys56), Ap2 (Cys110-Cys193, Cys136-Cys165, Cys140-Cys146), Ap3 (Cys200-Cys283, Cys226-Cys255, Cys230-Cys236) and Ap4 (Cys291-Cys374, Cys317-Cys346, Cys321-Cys327). Type I variants are circled in red, Type II in green and those of unknown phenotype in orange. Non-disease associated polymorphisms are circled in purple. The green residues in Ap4 mark those present at the FXI dimer interface. The ‘Sec’ rows highlight the α-helix region (H) and the five β-strand regions (E) in the consensus Ap domain. The β-strand regions are denoted AB, C, D, E and FG. The ‘Acc’ row denotes the relative accessibility of each consensus residue, where accessibilities of 0 or 1 indicate buried sidechains and accessibilities > 1 indicate sidechain exposure to solvent. (b) Variants are colored according to phenotype. Type I variants are colored in red, Type II in green and those of unknown phenotype in yellow. Polymorphisms are shown in purple. The sphere color represents the most commonly occurring phenotype at that position according to the sequence alignment in (a). The size of the spheres indicates the number of variants found at that position, and ranges from one to five. N and C refer to the N- and C-termini respectively. (c) Cys variants and their neighboring residues are shown as dark and light pink spheres respectively. All other variants are depicted in blue. The size of the spheres is again indicative of the number of variants found at that position. The six Cys residues are labeled C1-C6. ( d ) The FXI dimer interface is highlighted in green and the β-strands present at the interface are labeled. Variants found within Ap4 are shown as spheres, colored according to their phenotype as in (b).
