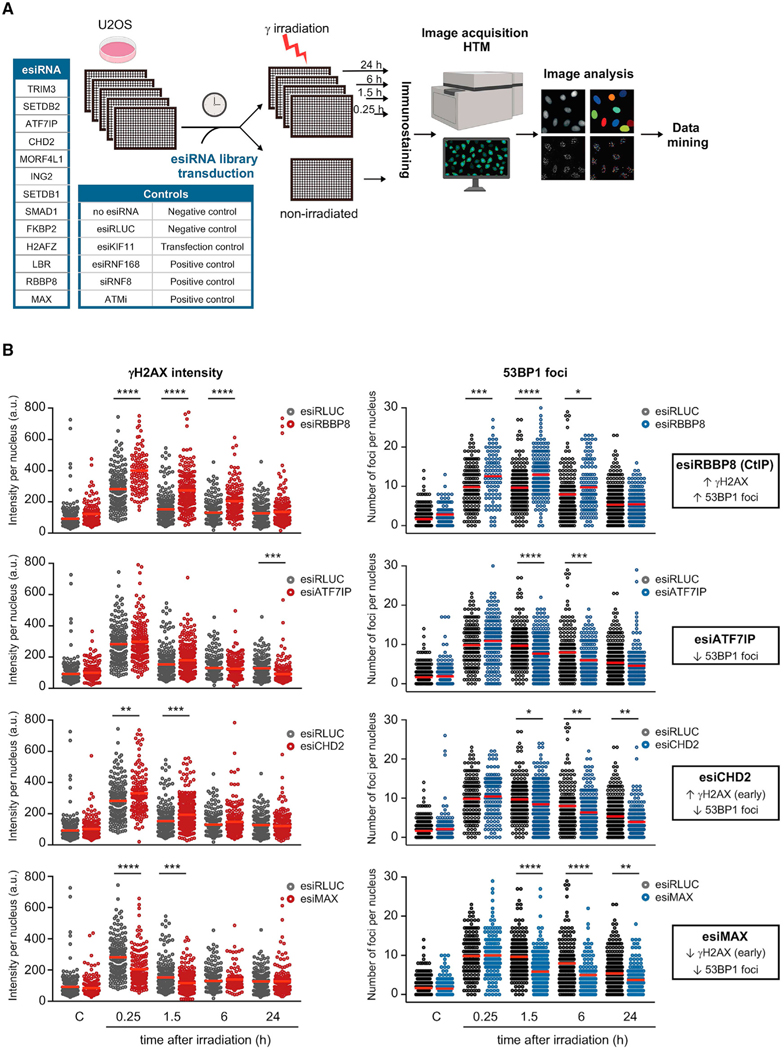
Figure 3. Downregulation of hits identified in the ChromORFeome HCS impacts DNA repair kinetics.
(A) Schematic of the esiRNA small screen for DNA repair. An esiRNA library containing esiRNA targeting 13 hits identified in our HCS for DNA repair was transduced in U2OS cells. Untransduced U2OS cells and U2OS cells transduced with an esiRNA targeting Renilla Luciferase (esiRLUC) were used as negative controls. esiRNA against human KIF11 inducing mitotic arrest was used as positive control of transfection. Transfection of U2OS cells with esiRNA targeting RNF168 or siRNA targeting RNF8, as well as treatment with the ATM inhibitor KU55933 (10 μM, 2 h prior to irradiation), were used as positive controls in the assay.
(B) Relative intensity of γH2AX staining per nucleus and number of 53BP1 foci per nucleus were determined at the indicated times after DNA damage (3 Gy) in U2OS cells transfected with the negative control esiRNA (RLUC) or with esiRNA targeting a particular chromatin factor identified by ML in the HCS for DNA repair. The impact in DNA repair kinetics of the downregulation of four hits (RBBP8, ATF7IP, CHD2, MAX) compared to control cells (esiRLUC) is shown: increased γH2AX intensity and increased number of 53BP1 foci after damage (esiRBBP8), decreased 53BP1 foci after damage (esiATF7IP), increased γH2AX intensity at early time points after damage, and decreased 53BP1 foci after damage (esiCHD2) and decreased γH2AX intensity and 53BP1 foci after damage (esiMAX). Red bars represent the mean. (*p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001 by one-way ANOVA with multiple comparisons, for each time point vs. esiRLUC).