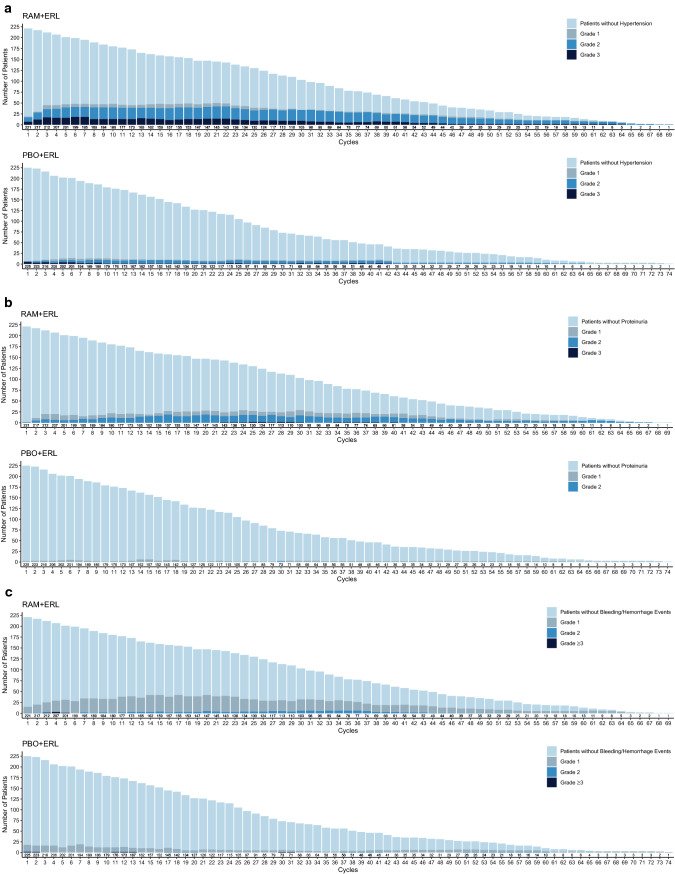
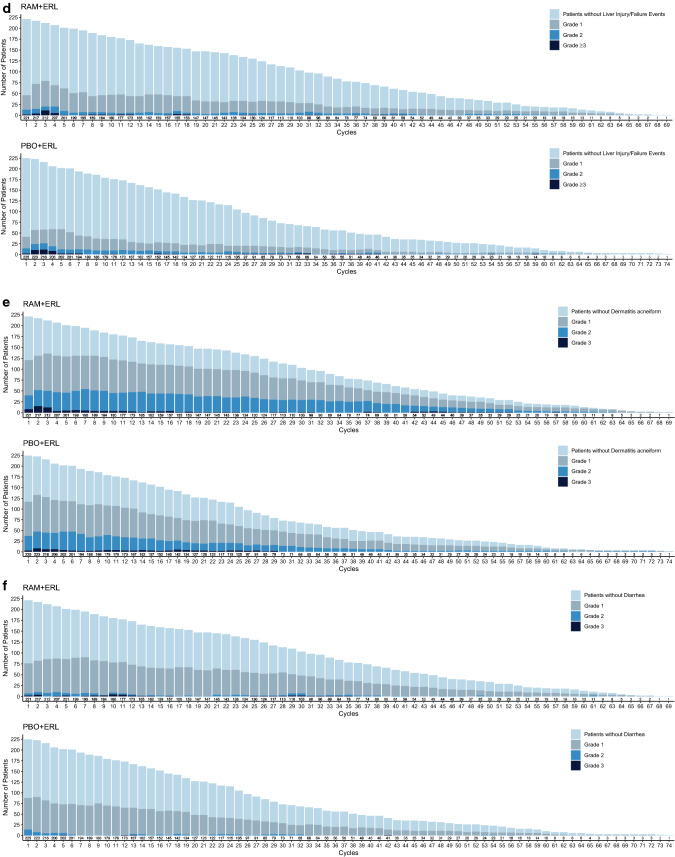
Fig. 4.
Number of patients experiencing adverse events of special interest (all grades) over time in RELAY: a hypertension, b proteinuria, c bleeding/hemorrhage, d hepatic events, e dermatitis, and f diarrhea. The frequencies of selected adverse events across treatment cycles were plotted by treatment arm using a stacked bar chart, where each bar represents one cycle and comprises four mutually exclusive groups: patients without events and patients with events of maximum grades 1, 2, or ≥ 3 in a given cycle. Plots are presented by treatment arm. ERL erlotinib, PBO placebo, RAM ramucirumab