Abstract
Background
Persistent immune activation due to gut barrier dysfunction is a suspected cause of morbidity in HIV, but the impact of menopause on this pathway is unknown.
Methods
In 350 women with HIV from the Women’s Interagency HIV Study, plasma biomarkers of gut barrier dysfunction (intestinal fatty acid binding protein; IFAB), innate immune activation (soluble CD14 and CD163; sCD14, sCD163), and systemic inflammation (interleukin-6 and tumor necrosis factor receptor 1; IL-6, TNFR1) were measured at 674 person-visits spanning ≤2 years.
Results
Menopause (post- vs premenopausal status) was associated with higher plasma sCD14 and sCD163 in linear mixed-effects regression adjusting for age and other covariates (β = 161.89 ng/mL; 95% confidence interval [CI], 18.37–305.41 and 65.48 ng/mL, 95% CI, 6.64–124.33, respectively); but not with plasma IFAB, IL-6, or TNFR1. In piece-wise linear mixed-effects regression of biomarkers on years before/after the final menstrual period, sCD14 increased during the menopausal transition by 250.71 ng/mL per year (95% CI, 16.63–484.79; P = .04), but not in premenopausal or postmenopausal periods.
Conclusions
In women with HIV, menopause may increase innate immune activation, but data did not support an influence on the gut barrier or inflammation. Clinical implications of immune activation during menopausal transition warrant further investigation.
Keywords: HIV, immune activation, inflammation, menopause, soluble CD14, soluble CD163
In women living with HIV on antiretroviral therapy, menopause was associated with increased levels of innate immune activation plasma biomarkers soluble CD14 and CD163, but not with biomarkers of gut barrier dysfunction or systemic inflammation.
Human immunodeficiency virus (HIV) infection leads to persistent immune activation and inflammation even with antiretroviral therapy (ART), which may contribute to higher risk of non-AIDS–related conditions (eg, cardiovascular disease [CVD], cancer) [1]. There are several hypothesized causes of immune activation in HIV, including low-level viral replication, coinfections, lymphoid fibrosis, and microbial translocation [2]. Microbial translocation is a process in which damage to the gut epithelial barrier, caused by HIV infection, allows microbial products to translocate from gut to circulation, activating an innate immune response [3]. Gut barrier dysfunction and innate immune activation have been associated with CVD and mortality in treated HIV infection [4–10]. Understanding drivers of immune activation and inflammation in HIV is important for prevention of non-AIDS–related diseases [11].
A recent report of 65 women without HIV found that biomarkers of gut barrier dysfunction, microbial translocation, and immune activation increased during the menopausal transition [12]. Menopause marks the end of the reproductive phase of a woman’s life, when complete depletion of ovarian follicles leads to loss of ovarian production of sex hormones estradiol and progesterone [13]. Both hormones appear important for gut barrier integrity [14–18], and estradiol also has well known cardioprotective properties [19–21]. In the context of HIV infection, estradiol and progesterone can inhibit HIV replication and are thought to maintain HIV latency [22, 23]. Women with HIV have lower estradiol and ovarian reserve [24, 25], higher levels of microbial translocation and immune activation [26], and higher cardiovascular risks [27–29] than women without HIV, suggesting that menopause-related decline in sex hormones may pose unique morbidity risks. However, the effect of the menopausal transition on microbial translocation, immune activation, and inflammation is unknown in women with HIV.
Here, we examined the association of menopause status with plasma biomarkers of gut barrier dysfunction, innate immune activation, and systemic inflammation in women with HIV from the Women’s Interagency HIV Study (WIHS). Additionally, we explored changes in these biomarkers over time relative to the final menstrual period, to understand effects of the menopausal transition above those of chronological aging alone [30].
METHODS
Study Population
The WIHS was a multicenter cohort of women with and without HIV in the United States that collected clinical, demographic, and behavioral data semiannually through interviews, physical examinations, and laboratory tests [31]. The present study is nested within a WIHS substudy [32, 33] that measured plasma biomarkers in women with HIV on ART from 10 sites (Bronx, NY; Brooklyn, NY; Washington, DC; San Francisco, CA; Chicago, IL; Chapel Hill, NC; Atlanta, GA; Miami, FL; Birmingham, AL; and Jackson, MI), without hepatitis B surface antigenemia or active hepatitis C virus infection at the time of enrollment into WIHS, and without self-reported cancer or autoimmune disease [33]. Biomarkers were measured on annual (longitudinal) samples collected from 2013 to 2015 for women from the northern sites (Bronx, Brooklyn, DC, San Francisco, Chicago; up to 3 samples per woman); or on a single sample collected in 2015 for women from the southern sites (Chapel Hill, Atlanta, Miami, Birmingham, Jackson). Thus, the number of samples per participant ranged from 1 to 3. Exclusions were performed at the level of the person-visit: we excluded person-visits with pregnancy, hormone replacement therapy in the past 6 months, hormonal contraceptive use during postmenopause, uncertain menopause status, and person-visits following a hysterectomy or oophorectomy that precluded a natural menopause (further description below). A total of 674 person-visits from 350 women contributed to the analysis (171, 34, and 145 participants provided 1, 2, and 3 person-visits, respectively). This research was approved by the Institutional Review Boards of all WIHS sites, and was conducted in accordance with the Declaration of Helsinki. Participants provided written informed consent.
Definition of Menopause Status
We used each participant’s longitudinal survey history since their enrollment in the WIHS to identify if/when they reached their natural final menstrual period, and accordingly classify women as premenopausal or postmenopausal at each WIHS visit. We also used the longitudinal history to identify if/when a participant had a surgical menopause, to exclude visits after surgical menopause from analysis. Menopause status was assessed semiannually with: “Have you been through menopause (the change of life)?” defined to participants as not having menstruated for 12 or more months. For participants who were postmenopausal at their most current visit, we obtained the date of the final menstrual period from the “first day of most recent period” at their last premenopausal study visit; or, if the date was missing, from the midpoint date of the time interval in which they had their last period (ie, within last month, 1–3 months, 3–6 months, etc.); or if that was also missing, we used the date of the last premenopausal visit. All visits prior to the final menstrual period were considered premenopausal, while all visits after the final menstrual period (ie, including the final premenopausal visit) were considered postmenopausal. For participants who were premenopausal at their most current visit, final menstrual period was not captured and all prior visits were considered premenopausal. If a woman reported surgery (hysterectomy or oophorectomy), all visits after the surgery date or the visit when surgery was first reported were coded as missing menopause status, unless the surgery occurred at least 2 years after a natural final menstrual period was captured. Lastly, if a woman reported menopause and later reported being premenopausal, visits between the first report of menopause and the last reported premenopausal visit were coded as missing due to uncertainty. Person-visits missing menopause status, as defined here, were excluded from analysis.
Covariate Data
Variables considered for inclusion in statistical models are shown in Table 1. Covariates were allowed to vary by person-visit within participants, excepting static variables (eg, race).
Table 1.
Characteristics of the Study Participants by Menopause Statusa
| Characteristic | Premenopause (n = 257) | Postmenopause (n = 93) | Standardized Mean Difference |
|---|---|---|---|
| Age, y, mean (SD) | 40.4 (6.4) | 53.0 (4.5) | 2.27 |
| Race, % | 0.22 | ||
| Black/African-American | 70.0 | 60.2 | |
| White | 9.3 | 15.1 | |
| Other or multiracial | 20.6 | 24.7 | |
| Hispanic, % | 9.7 | 16.1 | 0.19 |
| Country of birth, % | 0.30 | ||
| United States | 77.4 | 82.8 | |
| Puerto Rico or other US territories | 0.4 | 3.2 | |
| Other countries | 22.2 | 14.0 | |
| Education, % | 0.21 | ||
| Less than high school | 27.2 | 33.3 | |
| Completed high school | 28.0 | 32.3 | |
| Some college | 33.9 | 25.8 | |
| Completed college or any graduate school | 10.9 | 8.6 | |
| Employed, % | 44.7 | 38.7 | 0.12 |
| Alcohol use, % | 0.35 | ||
| Abstainer | 47.1 | 63.4 | |
| >0–7 drinks/wk | 44.4 | 29.0 | |
| >7–12 drinks/wk | 3.9 | 4.3 | |
| >12 drinks/wk | 4.7 | 3.2 | |
| Cigarette smoking history, % | 0.35 | ||
| Never smoker | 49.0 | 32.3 | |
| Current smoker | 28.0 | 36.6 | |
| Former smoker | 23.0 | 31.2 | |
| Drug use, %b | 16.3 | 20.4 | 0.11 |
| Hormonal contraceptive use, % | 13.2 | 0 | 0.55 |
| HIV load, copies/mL, mean (SD) | 4394.3 (26 608.4) | 211.0 (1313.8) | 0.22 |
| Undetectable HIV load, % | 76.7 | 83.9 | 0.18 |
| CD4 count, cells/mm3, mean (SD) | 648.9 (307.7) | 663.8 (338.2) | 0.05 |
| CD4 count ≥500 cells/mm3, % | 68.9 | 62.4 | 0.14 |
| BMI, kg/m2, mean (SD) | 33.2 (9.7) | 30.9 (8.0) | 0.25 |
| BMI category, % | 0.25 | ||
| Underweight or healthy weight, <25 kg/m2 | 16.0 | 23.7 | |
| Overweight, ≥25 and <30 kg/m2 | 26.1 | 30.1 | |
| Obese, ≥30 kg/m2 | 58.0 | 46.2 | |
| Diabetes, %c | 10.9 | 19.4 | 0.24 |
| Hypertension, %d | 31.1 | 53.8 | 0.47 |
Abbreviations: BMI, body mass index; HIV, human immunodeficiency virus.
aData are presented for the first visit with biomarker data. Missing data for covariates were imputed based on the immediately prior study visit with the data, if available (missingness was <1% for all covariates).
bInjected or noninjected drug use including marijuana.
cEver any fasting glucose ≥126 mg/dL, hemoglobin A1C ≥6.5%, self-reported diabetes, or diabetes medication.
dSystolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, self-report, or use of antihypertensive medication.
Biomarker Measurement
Enzyme-linked immunosorbent assays (R&D Systems) were used to assess plasma biomarkers of gut barrier dysfunction (intestinal fatty acid binding protein; IFAB), innate immune activation (soluble CD14 and CD163; sCD14, sCD163), and systemic inflammation (interleukin-6 and tumor necrosis factor receptor 1; IL-6, TNFR1) at Columbia University’s Irving Institute for Clinical and Translational Research, Biomarkers Core lab. All samples were stored at −80°C prior to analysis. Assays were performed in duplicate with positive and negative controls on each plate. Six samples below lower limit of detection (LOD) for sCD14 were assigned a value of LOD/2. Five samples above upper limit of detection for sCD163 were assigned the upper limit value. Two samples missing IL-6 and TNFR1 were excluded from analysis of these outcomes.
Statistical Analysis
General Principles
Longitudinal stability of biomarkers was assessed using intraclass correlation coefficients [34]. Biomarkers with right skew (IFAB, IL-6, TNFR1) were log transformed for regression analyses. Characteristics were compared between pre- and postmenopausal women at the first biomarker visit (ie, baseline visit) using standardized mean differences. All analyses were conducted using R 4.0.3.
Association of Menopause Status With Biomarker Outcomes
We used linear mixed effects models (lme4 package, R) to examine the association of menopause status (post- vs premenopause) with the continuous biomarker outcomes: log(IFAB), sCD14, sCD163, log(IL-6), and log(TNFR1). A random intercept was included to account for within-subject correlation. We developed nested models to serially adjust for potential confounders: model 1, unadjusted; model 2, age only; model 3, age, race, ethnicity, hormonal contraceptive use, HIV load, and CD4 count; model 4–model 3, variables plus any variables associated with menopause status (standardized mean difference >0.1). Because pre- and postmenopausal women differ in age, we also ran these models in a sensitivity analysis, in a subgroup of pre- and postmenopausal person-visits matched on age. We used nearest neighbor matching of the propensity score, with a caliper of 1 standard deviation (MatchIt package, R).
Biomarker Outcomes Relative to Time of Final Menstrual Period
The purpose of this analysis was to differentiate whether biomarkers increase at a constant rate over time, or accelerate in increase during the menopausal transition, suggesting an effect of ovarian versus chronological aging. We used a previously described [30] 2-stage approach in women with an observed final menstrual period, involving: (1) selection of knots from nonparametric locally weighted scatter plot smoothing curves of each biomarker trajectory over time since final menstrual period, and (2) piece-wise mixed effects linear regression with fixed knots to estimate biomarker change during each trajectory phase. In more detail, inflection points associated with increase or decrease of biomarkers around the final menstrual period were chosen visually from smoothing curves; or, if no obvious inflections were observed, default knots of 1 year before/after the final menstrual period were used based on previous literature [30]. Using these knots, time was divided into 3 segments: premenopausal, menopausal transition, and postmenopausal segments. Next, we fit 2 models for each biomarker as a function of time since final menstrual period: a linear mixed effects model, which assumes a constant slope across the 3 time segments; and a piece-wise mixed effects linear model, which allows slope to differ across the segments. The model does not force the 3 segments to connect, allowing more flexibility to accommodate distinct a priori stages of women’s reproductive life (although goodness of fit was similar in connected models). A random intercept was included. We considered models unadjusted for covariates, as well as adjusted for age at final menstrual period, race, ethnicity, hormonal contraceptive use, HIV load, CD4 count, and other variables associated with menopause status (standardized mean difference >0.1). The Akaike information criterion (AIC) was used to assess model fit, and the likelihood ratio test (LRT) was used to compare nested models (linear vs piece-wise). For interpretation, a similar biomarker change (slope) across all 3 time segments was considered consistent with chronological aging, but a difference in slope across segments, steeper during the menopausal transition compared to pre- or postmenopausal segments, was considered consistent with changes driven by ovarian aging [30]. For visualization, we plotted annual biomarker means over time before/after the final menstrual period, alongside estimates from piece-wise linear models.
RESULTS
Participant Characteristics
Of 350 women included in this analysis, 257 were premenopausal and 93 were postmenopausal at the baseline biomarker visit, with 8 switching from pre- to postmenopausal status over the 2 year follow-up of the biomarker study. Average age was 40 and 53 years for pre- and postmenopausal women, respectively. The majority of women identified as black (67%), were born in the United States (79%), and were obese (55%). All were on ART, with 79% virally suppressed and 67% with normal CD4 cell count (≥500 cells/mm3) at the baseline biomarker visit (Table 1). Women with 2–3 biomarker samples differed from women with only 1 sample on demographic characteristics, as expected given differences between northern and southern study sites; however, the proportion of postmenopausal women was the same (Supplementary Table 1). Within women providing longitudinal samples over up to 3 visits spanning up to 2 years, consistency was good for sCD163, moderate for IFAB, and poor for sCD14, IL-6, and TNFR1 (Table 2).
Table 2.
Intraclass Correlation Coefficients for Biomarkers of Gut Barrier Dysfunction, Innate Immune Activation, and Inflammation, Across up to 2 Years With up to 3 Visits per Participant
| Biomarker | n Women (n Person-Visits) | Intraclass Correlation Coefficient (95% CI) |
|---|---|---|
| log(IFAB, pg/mL) | 350 (674) | 0.57 (.49–.64) |
| sCD14, ng/mL | 350 (674) | 0.22 (.11–.32) |
| sCD163, ng/mL | 350 (674) | 0.81 (.77–.84) |
| log(IL-6, pg/mL) | 349 (672) | 0.20 (.10–.31) |
| log(TNFR1, pg/mL) | 350 (672) | 0.35 (.25–.44) |
Abbreviations: CI, confidence interval; IFAB, intestinal fatty acid binding protein; IL-6, interleukin 6; TNFR1, tumor necrosis factor receptor 1.
Association of Menopause Status and Biomarker Outcomes
For this analysis, we included all women providing at least 1 (but up to 3) person-visits with menopause and biomarker data (up to 350 women; up to 674 person-visits). Menopause status was associated with plasma sCD14 and sCD163 in linear mixed effects models adjusted for age, race, ethnicity, hormonal contraceptive use, HIV load, CD4 cell count, country of birth, educational attainment, employment status, alcohol use, smoking status, drug use, body mass index (BMI), diabetes, and hypertension (Table 3). Postmenopausal women had an estimated 161.89 ng/mL (95% CI, 18.37–305.41) and 65.48 ng/mL (95% CI, 6.64–124.33) higher plasma sCD14 and sCD163, respectively, than premenopausal women (P = .03 for both). A significant association of menopause status with plasma IFAB was attenuated upon adjustment for age, while menopause status was not associated with plasma IL-6 or TNFR1 in unadjusted or adjusted models (Table 3). Results were similar in a sensitivity analysis matching pre- and postmenopausal person-visits on age (Supplementary Table 2), despite reduced sample size due to exclusion of person-visits that could not be matched on age. Results were also similar in sensitivity analyses excluding premenopausal person-visits for women that later had surgical menopause, or excluding premenopausal person-visits for women who did not have a menstrual period within the last 3 months (Supplementary Tables 3 and 4). Interestingly, the association of menopause with sCD14 was only observed among women with controlled HIV (undetectable viral load and CD4 count ≥ 500 cells/mm3; P interaction = .03; Supplementary Table 5).
Table 3.
Association of Menopause Status (Post- vs Premenopause) With Biomarkers of Gut Barrier Dysfunction, Innate Immune Activation, and Inflammation in Linear Mixed Effects Models
| Model | log(IFAB, pg/mL) | sCD14, ng/mL | sCD163, ng/mL | log(IL-6, pg/mL) | log(TNFR1, pg/mL) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| β (95% CI) | P | β (95% CI) | P | β (95% CI) | P | β (95% CI) | P | β (95% CI) | P | |
| 1 | 0.29 (.15 to .42) | <.01 | 175.08 (64.86 to 285.3) | <.01 | 36.46 (−12.99 to 85.9) | .15 | 0.09 (−.05 to .24) | .20 | 0.06 (−.02 to .13) | .12 |
| 2 | 0.09 (−.08 to .27) | .30 | 146.45 (−2.19 to 295.1) | .05 | 50.69 (−10.85 to 112.23) | .11 | −0.04 (−.23 to .15) | .71 | 0.04 (−.06 to .14) | .40 |
| 3 | 0.10 (−.08 to .28) | .27 | 175.25 (30.21 to 320.28) | .02 | 55.94 (−3.07 to 114.95) | .06 | −0.03 (−.22 to .16) | .75 | 0.05 (−.04 to .15) | .28 |
| 4 | 0.07 (−.10 to .25) | .41 | 161.89 (18.37 to 305.41) | .03 | 65.48 (6.64 to 124.33) | .03 | −0.01 (−.18 to .17) | .93 | 0.06 (−.04 to .15) | .24 |
Abbreviations: BMI, body mass index; CI, confidence interval; HIV, human immunodeficiency virus; IFAB, intestinal fatty acid binding protein; IL-6, interleukin 6; TNFR1, tumor necrosis factor receptor 1.
All models include a random intercept. Model 1 is unadjusted for covariates; model 2 adjusts for age only; model 3 adjusts for age, race, ethnicity, hormonal contraceptive use, HIV load, and CD4 cell count; model 4 adjusts for all variables in model 3 plus country of birth, alcohol use, smoking status, drug use, educational attainment, employment status, BMI, diabetes, and hypertension. Number of unique IDs and person-visits, respectively, included in each model are: IFAB, sCD14, sCD163 (350, 674), IL-6 (349, 672), TNFR1 (350, 672). β represents difference in each outcome for post- compared to pre-menopausal women.
Association of Time Before/After the Final Menstrual Period With Biomarker Outcomes
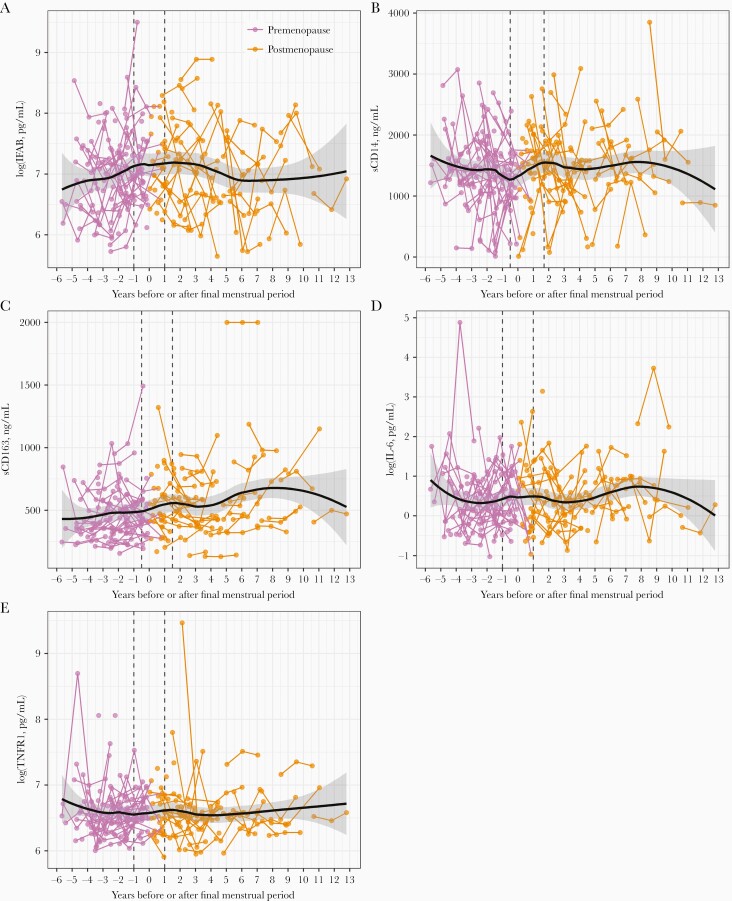
For this analysis, we included a subset of 126 women who had a prospectively observed final menstrual period (ie, gone through the menopausal transition) during their participation in the WIHS, which ranged from 3 to 25 (median 17) years; these women provided 286 biomarker person-visits. Premenopausal women in this subset tended to be older than those in the full dataset (Supplementary Table 6), and there was a strong correlation between age and time before/after the final menstrual period (r = 0.66, P < .0001). Nonparametric locally weighted scatter plot smoothing curves suggested that the change (slope) of plasma sCD14 and sCD163 over time relative to the final menstrual period, may differ during the menopausal transition compared with pre- or postmenopausal periods (Figure 1). Inflection points (knots) for piece-wise linear mixed effects models were chosen visually for sCD14 (0.5 years before/1.7 years after final menstrual period) and sCD163 (0.5 years before/1.5 years after final menstrual period). Default points of 1 year before/after the final menstrual period [30] were used for plasma IFAB, IL-6, and TNFR1 due to lack of obvious change in slope around the final menstrual period.
Figure 1.
Scatter plots of gut barrier dysfunction, innate immune activation, and inflammation biomarkers over years before/after the final menstrual period. In scatter plots of (A) log(IFAB), (B) sCD14, (C) sCD163, (D) log(IL-6), and (E) log(TNFR1), lines connect points from the same woman, and points/lines are colored according to menopause status. Plots are overlaid with nonparametric locally weighted smoothing curves, shown with black curves and 95% confidence intervals. Inflection points used in piece-wise linear mixed effects models are shown with dashed vertical lines; points were visually chosen for sCD14 and sCD163, while default points of 1 year before and 1 year after the final menstrual period were used for IFAB, IL-6, and TNFR1 due to lack of obvious change in slope around the final menstrual period. Number of unique identities and person-visits, respectively, included in each plot are: IFAB, sCD14, and sCD163, 350, 674; IL-6, 349, 672; and TNFR1, 350, 672. Abbreviations: IFAB, intestinal fatty acid binding protein; IL-6, interleukin 6; sCD14, soluble CD14; sCD163, soluble CD163; TNFR1, tumor necrosis factor receptor 1.
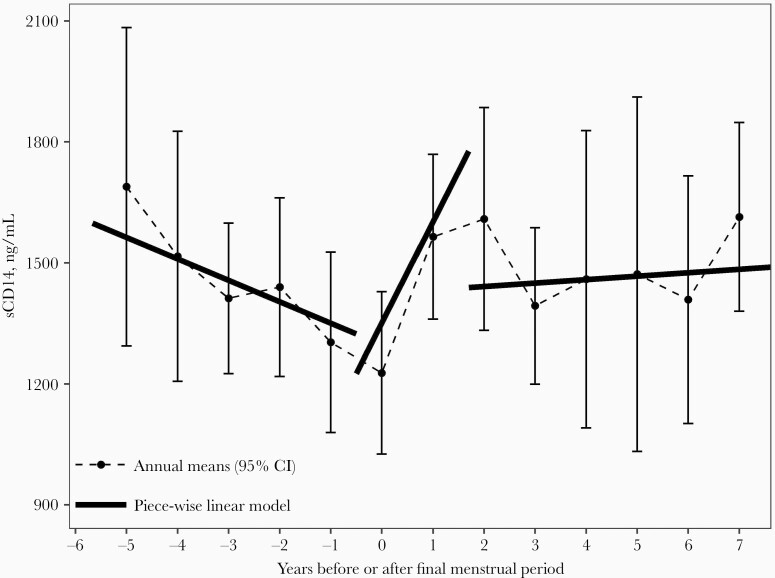
In piece-wise linear mixed effects models, we observed a significant increase in sCD14 during the menopausal transition (within 0.5 years before and 1.7 years after the final menstrual period); for each year in this period, sCD14 increased by 250.71 ng/mL (95% CI, 16.63–484.79; P = .04). The slope of sCD14 in the menopausal transition period differed from the premenopausal (>0.5 years before final menstrual period) and postmenopausal (>1.7 years after final menstrual period) periods (Table 4 and Figure 2). Additionally, the piece-wise linear model for sCD14 provided a marginally better fit than a linear model (piece-wise AIC = 4420, linear AIC = 4443, LRT P = .06). Results were similar adjusting for covariates (Table 4). For other outcomes (plasma IFAB, sCD163, IL-6, and TNFR1), we did not observe associations with time before/after the final menstrual period in piece-wise models.
Table 4.
Association of Years Before/After Final Menstrual Period With sCD14 (ng/mL) in Linear and Piece-Wise Linear Mixed Effects Models,a to Distinguish Effects of Chronological and Ovarian Aging
| Unadjusted | Multivariate Adjusted | |||||
|---|---|---|---|---|---|---|
| β (95% CI) | P Value | AIC | β (95% CI) | P Value | AIC | |
| Linear model | 4.92 (−37.48 to 47.31) | .82 | 4443.21 | 0.58 (−44.04 to 45.20) | .98 | 4241.58 |
| Piece-wise modelb | … | 4420.31 | … | 4216.18 | ||
| Slope in segment 2, menopausal transition | 250.71 (16.63 to 484.79) | .04 | … | 300.13 (56.75 to 543.51) | .02 | … |
| Slope difference, segment 1 vs 2 | −303.80 (−557.27 to −50.34) | .02 | … | −375.54 (−640.18 to −110.90) | .01 | … |
| Slope difference, segment 3 vs 2 | −242.19 (−483.18 to −1.20) | .05 | … | −294.04 (−545.14 to −42.93) | .02 | … |
| LRTc | … | .06 | … | … | 0.01 |
Abbreviations: AIC, Akaike information criterion; LRT, likelihood ratio test.
aAll models include a random intercept. Linear model includes terms for time since final menstrual period and period; piece-wise model includes terms for time since final menstrual period, period, and time*period. β represents change in sCD14 (ng/mL) per year. Adjusted model adjusts for age at final menstrual period, race, ethnicity, hormonal contraceptive use, HIV load, CD4 cell count, country of birth, alcohol use, smoking status, drug use, educational attainment, employment status, BMI, diabetes, and hypertension. Included in each model were 126 unique IDs and 286 person-visits.
bThe piece-wise model allows for different slopes in 3 time segments: segment 1 (over 0.5 years before final menstrual period); segment 2, ie, menopausal transition (within 0.5 years before and 1.7 years after final menstrual period); and segment 3 (over 1.7 years after final menstrual period).
cLRT comparing the piece-wise and linear model.
Figure 2.
Means of sCD14 (ng/mL) in years before/after the final menstrual period. Estimates from unadjusted piece-wise linear mixed effects model (described in Table 4) are overlaid on the plot. Data include 126 unique identities and 286 person-visits. Abbreviations: CI, confidence interval; sCD14, soluble CD14.
Discussion
In this study of women with HIV, we observed higher levels of innate immune activation, measured by plasma sCD14 and sCD163, in postmenopausal compared to premenopausal women. Additionally, our results suggested that plasma sCD14 increases specifically during the menopausal transition, consistent with changes driven by ovarian rather than chronological aging. However, menopause was not associated with IFAB, a biomarker of gut barrier dysfunction, or IL-6 and TNFR1, biomarkers of systemic inflammation. Due to the short follow-up in this study of up to 2 years, we did not have sufficient sample size to explore within-woman change in biomarkers as women transition from pre- to postmenopause—only 8 women transitioned during follow-up. Nevertheless, our results suggest a risk of increased immune activation during the menopausal transition in women with HIV.
Plasma sCD14 and sCD163 are nonspecific markers of monocyte and macrophage activation [35, 36]. Functioning as cell surface receptors, CD14 and CD163 are released upon ligand binding and cell activation, resulting in their soluble forms. CD14 binds lipopolysaccharide (LPS), a cell wall component of gram-negative bacteria, as well as other bacterial and endogenous compounds [35]. Additionally, inflammatory cytokines IL-6 and IL-1β induce release of sCD14 [35], and sCD14 has been recognized as an acute phase protein [37]. CD163 is a receptor for hemoglobin-haptoglobin complexes, and also binds gram-positive and gram-negative bacteria [36]. Thus, sCD14 and sCD163 may indicate microbial translocation, but could also relate to other homeostatic or inflammatory processes. sCD14 and sCD163 have been associated with CVD risk [7–10] and mortality [4–6] in HIV, suggesting that menopause-related increases in sCD14 and sCD163 may have clinical consequences. For example, the magnitude of increase in sCD14 we observed per year during the menopausal transition is comparable to the difference in sCD14 observed between HIV patients who died and controls in a mortality study [5]. However, the observed increase in sCD14 during the menopausal transition did not appear to be maintained postmenopause—in our piece-wise linear regression, the trajectory in the postmenopausal period did not align with the accelerated trajectory during the menopausal transition (Figure 2); and, although sCD163 was elevated in post- compared to premenopausal women, the increase during the menopausal transition was not statistically significant in our piece-wise models. The menopausal transition itself is increasingly recognized as a pivotal time for CVD prevention [21]. Whether the sharp but fleeting observed increase in sCD14 during the menopausal transition in women with HIV is important for disease risk will need to be evaluated in future research.
A plausible mechanism for the observed menopause-related increase in innate immune activation may be preventive effects of sex hormones on gut permeability and microbial translocation. Experimental evidence indicates that estrogen and progesterone maintain the gut barrier [14–18], and in human studies progesterone has been inversely correlated with microbial translocation [38, 39]. Thus, declines in estrogen and progesterone during the menopausal transition could impair the gut barrier and increase microbial translocation and immune activation. Only 1 study has examined the effect of menopause on the gut barrier, microbial translocation, and immune activation: in 65 women without HIV from the Study of Women’s Health Across the Nation (SWAN), plasma IFAB, lipopolysaccharide binding protein (LBP), and sCD14 increased significantly within woman from pre- to postmenopause, while lower plasma estradiol was associated with higher IFAB and sCD14 [12]. Although that analysis did not adjust for concurrent age, it suggests that microbial translocation increases over the menopausal transition, related to sex hormone changes. In our analysis, we did not observe an association of menopause with plasma IFAB after adjustment for age. IFAB is a cytosolic protein in enteroctyes, found to a greater extent in the small intestine than the colon, that is released upon gut epithelial damage [40]. Sex hormones may modulate the gut barrier via other mechanisms not captured by the IFAB biomarker, such as through maintenance of tight junctions [41]. Thus, an effect of menopause on gut barrier dysfunction cannot be ruled out here.
Aside from effects of menopause on the gut barrier, there may be other mechanisms for menopausal effects on immune activation in HIV. Estradiol and progesterone can inhibit HIV replication and regulate HIV latency [22, 23], suggesting that HIV reactivation may occur during menopause and cause immune activation. The effect of menopause on sCD14 in our study was only observed in women with controlled HIV, suggesting that HIV control may be an important effect modifier although the mechanism is unclear. Estrogens also interact with the gut microbiota [42], which in turn may modulate host immunity in HIV [43, 44]. In a previous study in WIHS we observed that postmenopausal status in women with HIV was associated with increased Enterobacteriales [45], which may preferentially translocate from the gut to the circulation in HIV infection [46], and lead to immune activation [44]. Research incorporating the gut microbiome and biomarkers of microbial translocation will assist with connecting these factors during menopause.
We did not find an association of menopause with biomarkers of systemic inflammation: IL-6, an inflammatory cytokine, and TNFR1, receptor for the cytokine tumor necrosis factor-α (TNF-α). Prior studies in women without HIV have observed increases in inflammatory cytokines, including IL-6 and TNF-α, in post- compared to premenopausal women, although not always [47–49]. Our observation of menopause-related increases in immune activation, but not inflammation, obscures the consequences of our findings, as inflammation is typically a direct consequence of immune activation. Alternatively, it is possible that IL-6 and TNFR1 are not ideal biomarkers to capture an effect of menopause on inflammation in the context of women with HIV.
This study was strengthened by the large sample size, longitudinal data collection, and the well-characterized WIHS cohort providing detailed information on prospectively ascertained menopause and other covariates. We also utilized best statistical practices from the leading menopausal transition cohort study (SWAN) [30] to assess biomarker changes during the menopausal transition. We were limited by the short follow-up period (up to 2 years) for this biomarker study, which prevented us from having sufficient person-visits to determine within-woman changes as women shift from pre- to postmenopause. We also did not have available data on other biomarkers of gut barrier dysfunction and microbial translocation (eg, tight junction biomarkers, LPS, or LBP), which have been studied. Lastly, we did not have hormonal measures of menopause and were limited by self-report data of menopause and last menstrual period, which could result in misclassification.
In summary, postmenopausal women with HIV had evidence of increased innate immune activation relative to premenopausal women, with this increase occurring during the menopausal transition. However, menopause was not associated with increases in key markers of systemic inflammation, making the consequences of the menopause-related immune activation unclear. Additional longitudinal research following women with HIV through the pre- and postmenopausal stages is necessary to explore whether women who experience an increase in immune activation during menopause also experience increased inflammation and other disease risks. Compounded effects of HIV, aging, and menopause on immune activation could put aging women with HIV at particularly high risk of non-AIDS–related disease and mortality. Thus, research on the health of women with HIV during and after the menopausal transition is of paramount importance, including clinical research on the effectiveness of interventions (eg, health monitoring, lifestyle modifications, hormone therapy) to reduce disease risk.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgment . The authors gratefully acknowledge the contributions of the study participants and dedication of the staff at the MWCCS sites.
Disclaimer . The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH).
Financial support. This work was supported by the National Institute of Mental Health Women’s Interagency HIV Study (WIHS) substudy (grant number R01MH095683 to S. W.); the National Institute of Allergy and Infectious Diseases (grant number K24AI134326 to S. W.); and the National Heart, Lung, and Blood Institute (grant number R01HL148094 to R. C. K.).
Data in this article were collected by the WIHS, now the MACS/WIHS Combined Cohort Study (MWCCS). MWCCS (Principal Investigators), NHLBI grant numbers: Atlanta CRS (Ighovwerha Ofotokun, Anandi Sheth, and Gina Wingood), U01-HL146241; Baltimore CRS (Todd Brown and Joseph Margolick), U01-HL146201; Bronx CRS (Kathryn Anastos and Anjali Sharma), U01-HL146204; Brooklyn CRS (Deborah Gustafson and Tracey Wilson), U01-HL146202; Data Analysis and Coordination Center (Gypsyamber D’Souza, Stephen Gange and Elizabeth Golub), U01-HL146193; Chicago-Cook County CRS (Mardge Cohen and Audrey French), U01-HL146245; Chicago-Northwestern CRS (Steven Wolinsky), U01-HL146240; Northern California CRS (Bradley Aouizerat, Jennifer Price, and Phyllis Tien), U01-HL146242; Los Angeles CRS (Roger Detels and Matthew Mimiaga), U01-HL146333; Metropolitan Washington CRS (Seble Kassaye and Daniel Merenstein), U01-HL146205; Miami CRS (Maria Alcaide, Margaret Fischl, and Deborah Jones), U01-HL146203; Pittsburgh CRS (Jeremy Martinson and Charles Rinaldo), U01-HL146208; UAB-MS CRS (Mirjam-Colette Kempf, Jodie Dionne-Odom, and Deborah Konkle-Parker), U01-HL146192; UNC CRS (Adaora Adimora), U01-HL146194.
The MWCCS is funded primarily by the National Heart, Lung, and Blood Institute, with additional co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institute on Aging, National Institute of Dental and Craniofacial Research, National Institute of Allergy and Infectious Diseases, National Institute of Neurological Disorders and Stroke, National Institute of Mental Health, National Institute on Drug Abuse, National Institute of Nursing Research, National Cancer Institute, National Institute on Alcohol Abuse and Alcoholism, National Institute on Deafness and Other Communication Disorders, National Institute of Diabetes and Digestive and Kidney Diseases, and National Institute on Minority Health and Health Disparities, and in coordination and alignment with the research priorities of the NIH, Office of AIDS Research. MWCCS data collection is also supported by NIH grant numbers UL1-TR000004 to UCSF CTSA, UL1-TR003098 to JHU ICTR, UL1-TR001881 to UCLA CTSI, P30-AI-050409 to Atlanta CFAR, P30-AI-073961 to Miami CFAR, P30-AI-050410 to UNC CFAR, P30-AI-027767 to UAB CFAR, and P30-MH-116867 to Miami CHARM.
Potential conflicts of interest. N. S. is a consultant with Ansh Labs and ASTELLAS/Ogeda, and has received grant support from Menogenix, Inc, outside the submitted work. P. C. T. has received grant support from Merck, Gilead, and Eli Lilly outside the submitted work. A. A. A. has received personal funds for consulting from Merck, Viiv, and Gilead, and grants from Merck and Gilead outside the submitted work. A. S. has received grant funding from Gilead Sciences, Inc, outside the submitted work. M. L. A. has received personal fees from Merck outside the submitted work. All other authors declare no conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Lederman MM, Funderburg NT, Sekaly RP, Klatt NR, Hunt PW. Residual immune dysregulation syndrome in treated HIV infection. Adv Immunol 2013; 119:51–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hatano H. Immune activation and HIV persistence: considerations for novel therapeutic interventions. Curr Opin HIV AIDS 2013; 8:211–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Klatt NR, Funderburg NT, Brenchley JM. Microbial translocation, immune activation, and HIV disease. Trends Microbiol 2013; 21:6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hunt PW, Sinclair E, Rodriguez B, et al. . Gut epithelial barrier dysfunction and innate immune activation predict mortality in treated HIV infection. J Infect Dis 2014; 210:1228–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sandler NG, Wand H, Roque A, et al. ; INSIGHT SMART Study Group. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis 2011; 203:780–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Knudsen TB, Ertner G, Petersen J, et al. . Plasma soluble CD163 level independently predicts all-cause mortality in HIV-1-infected individuals. J Infect Dis 2016; 214:1198–204. [DOI] [PubMed] [Google Scholar]
- 7. Kelesidis T, Kendall MA, Yang OO, Hodis HN, Currier JS. Biomarkers of microbial translocation and macrophage activation: association with progression of subclinical atherosclerosis in HIV-1 infection. J Infect Dis 2012; 206:1558–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Longenecker CT, Jiang Y, Orringer CE, et al. . Soluble CD14 is independently associated with coronary calcification and extent of subclinical vascular disease in treated HIV infection. AIDS 2014; 28:969–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hanna DB, Lin J, Post WS, et al. . Association of macrophage inflammation biomarkers with progression of subclinical carotid artery atherosclerosis in HIV-infected women and men. J Infect Dis 2017; 215:1352–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Burdo TH, Lo J, Abbara S, et al. . Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J Infect Dis 2011; 204:1227–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Justice AC, Erlandson KM, Hunt PW, Landay A, Miotti P, Tracy RP. Can biomarkers advance HIV research and care in the antiretroviral therapy era?. J Infect Dis 2018; 217:521–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shieh A, Epeldegui M, Karlamangla AS, Greendale GA. Gut permeability, inflammation, and bone density across the menopause transition. JCI Insight 2020; 5:e134092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Santoro N. The menopausal transition. Am J Med 2005; 118 (Suppl 12B):8–13. [DOI] [PubMed] [Google Scholar]
- 14. Braniste V, Leveque M, Buisson-Brenac C, Bueno L, Fioramonti J, Houdeau E. Oestradiol decreases colonic permeability through oestrogen receptor beta-mediated up-regulation of occludin and junctional adhesion molecule-A in epithelial cells. J Physiol 2009; 587:3317–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Collins FL, Rios-Arce ND, Atkinson S, et al. . Temporal and regional intestinal changes in permeability, tight junction, and cytokine gene expression following ovariectomy-induced estrogen deficiency. Physiol Rep 2017; 5:e13263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Honda J, Iijima K, Asanuma K, et al. . Estrogen enhances esophageal barrier function by potentiating occludin expression. Dig Dis Sci 2016; 61:1028–38. [DOI] [PubMed] [Google Scholar]
- 17. van der Giessen J, van der Woude CJ, Peppelenbosch MP, Fuhler GM. A direct effect of sex hormones on epithelial barrier function in inflammatory bowel disease models. Cells 2019; 8:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhou Z, Bian C, Luo Z, et al. . Progesterone decreases gut permeability through upregulating occludin expression in primary human gut tissues and Caco-2 cells. Sci Rep 2019; 9:8367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Iorga A, Cunningham CM, Moazeni S, Ruffenach G, Umar S, Eghbali M. The protective role of estrogen and estrogen receptors in cardiovascular disease and the controversial use of estrogen therapy. Biol Sex Differ 2017; 8:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Clegg D, Hevener AL, Moreau KL, et al. . Sex hormones and cardiometabolic health: role of estrogen and estrogen receptors. Endocrinology 2017; 158:1095–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khoudary SRE, Aggarwal B, Beckie TM, et al. Menopause transition and cardiovascular disease risk: implications for timing of early prevention: a scientific statement from the American Heart Association. Circulation. p. 2020; 142:e506–32.. [DOI] [PubMed]
- 22. Devadas K, Biswas S, Ragupathy V, Lee S, Dayton A, Hewlett I. Modulation of HIV replication in monocyte derived macrophages (MDM) by steroid hormones. PLoS One 2018; 13:e0191916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Das B, Dobrowolski C, Luttge B, et al. . Estrogen receptor-1 is a key regulator of HIV-1 latency that imparts gender-specific restrictions on the latent reservoir. Proc Natl Acad Sci U S A 2018; 115:E7795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Karim R, Mack WJ, Kono N, et al. . Gonadotropin and sex steroid levels in HIV-infected premenopausal women and their association with subclinical atherosclerosis in HIV-infected and -uninfected women in the women’s interagency HIV study (WIHS). J Clin Endocrinol Metab 2013; 98:E610–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wessman M, Korsholm AS, Bentzen JG, et al. . Anti-Müllerian hormone levels are reduced in women living with human immunodeficiency virus compared to control women: a case-control study from Copenhagen, Denmark. J Virus Erad 2018; 4:123–7. [PMC free article] [PubMed] [Google Scholar]
- 26. Alcaide ML, Parmigiani A, Pallikkuth S, et al. . Immune activation in HIV-infected aging women on antiretrovirals—implications for age-associated comorbidities: a cross-sectional pilot study. PLoS One 2013; 8:e63804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab 2007; 92:2506–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hanna DB, Ramaswamy C, Kaplan RC, et al. . Sex- and poverty-specific patterns in cardiovascular disease mortality associated with human immunodeficiency virus, New York City, 2007–2017. Clin Infect Dis 2019 71:491-8.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stone L, Looby SE, Zanni MV. Cardiovascular disease risk among women living with HIV in North America and Europe. Curr Opin HIV AIDS 2017; 12:585–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Matthews KA, Crawford SL, Chae CU, et al. . Are changes in cardiovascular disease risk factors in midlife women due to chronological aging or to the menopausal transition?. J Am Coll Cardiol 2009; 54:2366–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Adimora AA, Ramirez C, Benning L, et al. . Cohort profile: the women’s interagency HIV study (WIHS). Int J Epidemiol 2018; 47:393–394i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Peters BA, Sheira LA, Hanna DB, et al. . Food insecurity and T-cell dysregulation in women living with HIV on antiretroviral therapy. Clin Infect Dis 2021; 72:e112–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Leddy AM, Roque A, Sheira LA, et al. . Food insecurity is associated with inflammation among women living with HIV. J Infect Dis 2019; 219:429–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med 2016; 15:155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shive CL, Jiang W, Anthony DD, Lederman MM. Soluble CD14 is a nonspecific marker of monocyte activation. AIDS 2015; 29:1263–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fabriek BO, van Bruggen R, Deng DM, et al. . The macrophage scavenger receptor CD163 functions as an innate immune sensor for bacteria. Blood 2009; 113:887–92. [DOI] [PubMed] [Google Scholar]
- 37. Bas S, Gauthier BR, Spenato U, Stingelin S, Gabay C. CD14 is an acute-phase protein. J Immunol 2004; 172:4470–9. [DOI] [PubMed] [Google Scholar]
- 38. Zhou Z, Powell AM, Ramakrishnan V, Eckard A, Wagner C, Jiang W. Elevated systemic microbial translocation in pregnant HIV-infected women compared to HIV-uninfected women, and its inverse correlations with plasma progesterone levels. J Reprod Immunol 2018; 127:16–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tremellen K, Syedi N, Tan S, Pearce K. Metabolic endotoxaemia—a potential novel link between ovarian inflammation and impaired progesterone production. Gynecol Endocrinol 2015; 31:309–12. [DOI] [PubMed] [Google Scholar]
- 40. Wells JM, Brummer RJ, Derrien M, et al. . Homeostasis of the gut barrier and potential biomarkers. Am J Physiol Gastrointest Liver Physiol 2017; 312:G171–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Grishina I, Fenton A, Sankaran-Walters S. Gender differences, aging and hormonal status in mucosal injury and repair. Aging Dis 2014; 5:160–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Baker JM, Al-Nakkash L, Herbst-Kralovetz MM. Estrogen-gut microbiome axis: physiological and clinical implications. Maturitas 2017; 103:45–53. [DOI] [PubMed] [Google Scholar]
- 43. Mutlu EA, Keshavarzian A, Losurdo J, et al. . A compositional look at the human gastrointestinal microbiome and immune activation parameters in HIV infected subjects. PLoS Pathog 2014; 10:e1003829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liu J, Williams B, Frank D, Dillon SM, Wilson CC, Landay AL. Inside out: HIV, the gut microbiome, and the mucosal immune system. J Immunol 2017; 198:605–14. [DOI] [PubMed] [Google Scholar]
- 45. Peters BA, Xue X, Wang Z, et al. . Menopausal status and observed differences in the gut microbiome in women with and without HIV infection. Menopause 2021; 28:491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Klase Z, Ortiz A, Deleage C, et al. . Dysbiotic bacteria translocate in progressive SIV infection. Mucosal Immunol 2015; 8:1009–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sites CK, Toth MJ, Cushman M, et al. . Menopause-related differences in inflammation markers and their relationship to body fat distribution and insulin-stimulated glucose disposal. Fertil Steril 2002; 77:128–35. [DOI] [PubMed] [Google Scholar]
- 48. McKane WR, Khosla S, Peterson JM, Egan K, Riggs BL. Circulating levels of cytokines that modulate bone resorption: effects of age and menopause in women. J Bone Miner Res 1994; 9:1313–8. [DOI] [PubMed] [Google Scholar]
- 49. Gameiro CM, Romão F, Castelo-Branco C. Menopause and aging: changes in the immune system—a review. Maturitas 2010; 67:316–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.