Graphical abstract
Keywords: Neuropeptide, Neuropeptide-like protein, NLP, Nematode, Parasite, Ascaris
Highlights
-
•
Parasites possess a reduced complement of Caenorhabditis elegans neuropeptide-like protein (NLP)-encoding genes.
-
•
Parasite NLP profiles are broadly conserved between nematode clades.
-
•
Five NLP-encoding genes are completely conserved in the nine parasitic nematodes examined.
-
•
Fourteen novel nematode NLP encoding genes are identified.
-
•
Several highly conserved NLPs are bioactive.
Abstract
Nematode parasite infections cause disease in humans and animals and threaten global food security by reducing productivity in livestock and crop farming. The escalation of anthelmintic resistance in economically important nematode parasites underscores the need for the identification of novel drug targets in these worms. Nematode neuropeptide signalling is an attractive system for chemotherapeutic exploitation, with neuropeptide G-protein coupled receptors (NP-GPCRs) representing the lead targets. In order to successfully validate NP-GPCRs for parasite control it is necessary to characterise their function and importance to nematode biology. This can be aided through identification of receptor activating ligand(s) via deorphanisation. Such efforts require the identification of all neuropeptide ligands within parasites. Here we mined the genomes of nine therapeutically relevant pathogenic nematodes to characterise the neuropeptide-like protein complements and demonstrate that: (i) parasitic nematodes possess a reduced complement of neuropeptide-like protein-encoding genes relative to Caenorhabditis elegans; (ii) parasite neuropeptide-like protein profiles are broadly conserved between nematode clades; (iii) five Ce-nlps are completely conserved across the nematode species examined; (iv) the extent and position of neuropeptide-like protein-motif conservation is variable; (v) novel RPamide-encoding genes are present in parasitic nematodes; (vi) novel Allatostatin-C-like peptide encoding genes are present in both C. elegans and parasitic nematodes; (vii) novel neuropeptide-like protein families are absent in C. elegans; and (viii) highly conserved nematode neuropeptide-like proteins are bioactive. These data highlight the complexity of nematode neuropeptide-like proteins and reveal the need for nomenclature revision in this diverse neuropeptide family. The identification of neuropeptide-like protein ligands, and characterisation of those with functional relevance, advance our understanding of neuropeptide signalling to support exploitation of the neuropeptidergic system as an anthelmintic target.
1. Introduction
Nematode parasites continue to threaten human health and global food security (Hotez et al., 2008, Payne and Fitchett, 2010, Elling, 2013, Roeber et al., 2013, Charlier et al., 2014, Mavrot et al., 2015, Coyne et al., 2018, Lopes-Caitar et al., 2019, Hotez and Lo, 2020). The impact of nematode parasites is exacerbated by the limited number of available anthelmintic drugs and the escalation of anthelmintic resistance in key nematode pathogens (Kaplan and Vidyashankar, 2012, Sangster et al., 2018). The disruption of nematode neuromuscular signalling is a well-established route to parasite control (McVeigh et al., 2006a, McVeigh et al., 2012, Li and Kim, 2008), where the neuropeptide signalling component represents an appealing unexploited source of novel anthelmintic targets.
Nematode neuropeptide families encompass the FMRF-amide like peptides (FLPs), neuropeptide-like proteins (NLPs) and the insulin-like peptides (INSs). Whilst INSs peptides appear to signal through receptor tyrosine kinases (Kimura et al., 1997), the majority of FLPs and NLPs are believed to act as ligands for G-protein coupled receptors (GPCRs) (Li and Kim, 2008). Within the neuropeptide system, neuropeptide GPCRs (NP-GPCRs) emerge as the most promising novel anthelmintic targets, with a proven history of ‘druggability’ (for review see McVeigh et al., 2012). Ultimately, validation of NP-GPCRs as therapeutic targets requires functional characterisation to enable the selection of the most appealing candidates.
While heterologous approaches to NP-GPCR deorphanisation in Caenorhabditis elegans have been successful, only a limited number of C. elegans NP-GPCRs have been functionally deorphanised, and only two reports of successful NP-GPCR deorphanisation have been described in parasites (Atkinson et al., 2013, Anderson et al., 2014). Whilst a major challenge of receptor deorphanisation is functional expression (McCoy et al., 2017), to aid deorphanisation efforts in therapeutically relevant nematode parasites we also require a complete profile of all potential interacting neuropeptide ligands and receptors. Recent studies have highlighted comprehensive analyses of FLP and NP-GPCR profiles in parasitic nematodes (McCoy et al., 2014), however our current understanding of NLP complements is significantly more limited (McVeigh et al., 2008, Buzy et al., 2021).
Here, we utilise an established neuropeptide Basic Local Alignment Search Tool (BLAST) approach to provide a comprehensive analyses of NLP-encoding gene profiles in nine key nematode parasites, and examine the bioactivity of the most highly conserved NLPs. These data advance understanding of nematode neuropeptide signalling and are critical to future deorphanisation efforts in parasitic nematodes that drive novel parasite control strategies.
2. Materials and methods
2.1. Species selection
Caenorhabditis elegans nlp gene sequelogues were identified in the genomic datasets of nine parasitic nematode species (Supplementary Table S1). The species employed in this study were selected based on a number of criteria including: (i) quality and availability of genomic and transcriptomic data, (ii) species employed in previously published neuropeptide analyses (for comparative purposes; (McCoy et al., 2014)), (iii) species appeal from a parasite control perspective (key parasites of humans, livestock and plants), (iv) representatives across clades and lifestyles, (v) species that are experimentally tractable and/or have been studied with respect to nematode neurobiology, and (iv) species for which we have amassed data on the localisation and function of neuropeptides.
2.2. BLAST searches
A reciprocal BLAST method was used to identify nlp gene sequelogues using the ‘prepropeptide search string’ approach (McCoy et al., 2014). Protein sequences for NLPs identified previously in C. elegans (Nathoo et al., 2001, Husson et al., 2005, Husson et al., 2007, Husson et al., 2014, McVeigh et al., 2008, Yamada et al., 2010, Howe et al., 2017, Van Bael et al., 2018; Supplementary Table S2) were obtained from WormBase (https://www.wormbase.org; WS276) and used as queries in translated nucleotide (tBLASTn) and predicted protein (BLASTp) searches of the genome datasets. tBLASTn searches were carried out on the WormBase ParaSite server (https://parasite.wormbase.org/index.html; Howe et al., 2017) ; BLASTp searches were carried out on the WormBase ParaSite Server or via a local Windows command line-based NCBI-BLAST. All BLAST searches were conducted between April 2017 and August 2018. Where there were multiple genomes available for a single species, all were mined (Supplementary Table S1). BLAST searches were also carried out in four additional Caenorhabditis spp. (Caenorhabditis brenneri, Caenorhabditis briggsae, Caenorhabditis remanei and Caenorhabditis japonica; as previously described (McCoy et al., 2014)). nlp genes (nlp-24–34), thought to have antimicrobial activity and distinguished by their glycine-rich sequences, were not included in this study due to their unsuitability for the BLAST search approach (McVeigh et al., 2008, Pujol et al., 2008, Dierking et al., 2016). Where nlp genes encoded multiple isoforms, only the longest were used as query sequences unless isoforms were significantly different. Expect values were set to ≥1000 to avoid false negative returns. All returned sequences were examined manually for NLP motifs flanked by putative mono/dibasic cleavage sites, signal peptide cleavage sites, or C-terminal signatures to eliminate false positive hits (McVeigh et al., 2008, McCoy et al., 2014) and peptides were predicted accordingly (see Supplementary Table S7) .
2.3. Post-BLAST analysis
Nematode parasite NLP-encoding gene sequelogues were assigned primarily based on sequence homology to the predicted peptide region of Ce-nlps (Li and Kim, 2008, Van Bael et al., 2018). Sequences were aligned using Vector NTI Advance 11.5 AlignX® multiple sequence alignment tool (Lu and Moriyama, 2004). Signal peptide predictions were generated using SignalP4.1 (Nielsen, 2017), however lack of a predicted signal peptide did not exclude NLP designation.
2.4. Clustered analysis of sequences (CLANS) analysis
The Clustered Analysis of Sequences (CLANS) algorithm was used to perform all-against-all BLASTp comparisons between the identified nematode RPamide and Allatostatin-C like prepropeptide sequences, and generate a three-dimensional (3D) similarity matrix (Frickey and Lupas, 2004). Prepropeptide sequences were analysed using the CLANS tool (https://toolkit.tuebingen.mpg.de/#/tools/clans) and the most appropriate E-value limit was determined to facilitate sufficient cluster separation. All other parameters were set as default. The CLANS file output was examined and coloured after 10,000 clustering rounds using the Java-based desktop software.
2.5. RNAseq analysis
Publicly available life-stage-specific transcriptomic data were downloaded from the WormBase ParaSite Gene Expression server (Howe et al., 2017). Raw sequence reads for Haemonchus contortus and Trichuris muris were downloaded using the NCBI SRA Toolkit (Leinonen et al., 2011). Forward and reverse fastq files were trimmed to remove adapter, leading, tailing and low quality sequences using Trimmomatic (v0.36; parameter: LEADING:5 TRAILING:5 SLIDINGWINDOW:3:15 MINLEN:34 (Bolger et al., 2014)). Appropriate genome assemblies were accessed via WormBase ParaSite FTP server for previous versions of WormBase ParaSite (Howe et al., 2017) and HISAT2 (v2.1.0 (Kim et al., 2015)) was employed to map trimmed reads to genomic data. Raw gene counts were assigned to mapped reads using SubRead v 2.0.1 featureCounts (Liao et al., 2014). Fragments per Kilobase of Transcript per Million (FPKM) data were generated via transformation of raw counts of orthologous genes using countToFPKM in R Studio and median FPKMs calculated.
2.6. Ascaris suum ovijector physiology assays
Selected Ascaris suum NLP peptides were tested for activity on the A. suum ovijector (reproductive muscle) using an established physiology assay (Fellowes et al., 1998, Fellowes et al., 2000, Moffett et al., 2003). Adult female A. suum (>20 cm) were collected from the intestines of pigs at a local abattoir (Karro, Northern Ireland) and transported back to the laboratory in mammalian saline (0.9% NaCl, 37 °C). Worms were maintained in Ascaris Ringers Solution (ARS; 13.14 mM NaCl, 9.47 mM CaCl2, 7.83 mM MgCl2, 12.09 mM C4H11NO3(Tris), 99.96 mM NaC2H3O2, 19.64 mM KCl, pH 7.8) at 37 °C and 5 % CO2 for up to 4 days with media changes twice daily. The ovijector was dissected from healthy, turgid worms and transferred to Hank’s balanced salt solution (HBSS; Life technologies) at 37 °C in a 4 ml recording chamber, where the tissue was attached between a flexible and an inflexible pipette. Tissue activity was amplified and recorded via a photo-optic transducer system (Fetterer et al., 1977, Marks et al., 1996). Ovijectors which displayed regular, spontaneous contractility were equilibrated for at least 5 min before peptide addition. Inactive or erratically active ovijectors were discarded. Peptides were synthesised (Genosphere Biotechnologies Inc.) and 10 mM stock solutions prepared in double distilled (dd)H2O, Dimethyl sulfoxide (DMSO), or Dimethyl formaldehyde (DMF) and stored in aliquots at −20 °C until use. Addition of 4 µl of ddH2O, DMSO or DMF had no effect on ovijector activity. Peptide was added to the 4 ml water bath such that addition of 4 µl gave a final peptide concentration of 10 µM. Peptide effects were recorded for 10 min before media were replaced with fresh HBSS. The ovijector was subsequently recorded for a further 10 min to observe recovery. Contraction amplitude and frequency were measured 2 min prior to: time 0 (peptide addition), 2, 5, 10 and 20 min post-addition. In some cases, it was necessary to analyse additional time points to capture transient effects. The change in tension was also measured at time 0, 2, 5, 10 and 20 min post-addition, where time 0 = 0 mg/mm. Note that muscle relaxation caused an increase in tension (+mg/mm; shortening of the tissue) whereas muscle contraction caused a decrease in tension (-mg/mm; lengthening of tissue). Data were statistically analysed (Graphpad Prism 8) with repeated measures ANOVA followed by Dunnett’s post-test to compare each time point with time 0.
3. Results and discussion
3.1. Parasitic nematodes possess a reduced complement of C. elegans NLP-encoding genes
nlp gene sequelogues (326) were identified in nine key nematode parasites (Table 1; Supplementary Fig. S1; Supplementary Table S3). These included 73 previously reported NLP-encoding genes identified through in silico and peptidomics studies (Nathoo et al., 2001, McVeigh et al., 2008, Jarecki et al., 2011, Koziol et al., 2016, Warnock et al., 2017, Van Bael et al., 2018, Buzy et al., 2021), and 253 novel sequences identified in this study. These data provide an insight into nlp conservation and diversity in nematode parasites that include multiple clades and lifestyles. As noted with parasite FLP and NP-GPCR datasets (McCoy et al., 2014) the parasitic nematode species investigated here displayed reduced complements of the Ce-NLP profile (Table 1). The human hookworm Necator americanus exhibited the largest share (67%) of Ce-nlps; this is unsurprising considering they are both members of clade 9 (Holterman et al., 2006). In contrast, the clade 2 species Trichinella spiralis and T. muris displayed a significantly reduced nlp complement of 15% and 14% of the Ce-nlp profile, respectively. This is likely a result of both specific gene loss in the clade 2 species, as well as nlp gene duplication events within the lineages that led to the extant nematodes comprising the ‘crown’ clades (Holterman et al., 2006) that exhibit broader nlp complements. In addition, RNASeq data show that almost all of the predicted nlps reported here are expressed in at least one of the parasitic nematodes examined and appear to be differentially expressed across lifecycle stages (see Supplementary Fig. S2). These data provide confidence that the nlps identified by our in silico approach have roles in nematode biology.
Table 1.
nlp-gene sequelogues in nine nematode parasite species. A black box indicates the presence of a sequelogue identified via BLAST search.
 |
aNovel gene identified in this study.
3.2. Parasite nlp profiles are more conserved in closely related species within the same clade
There appeared to be less intra-clade than inter-clade nlp profile variation, mirroring trends observed for flps and NP-GPCRs (Holterman et al., 2006, McCoy et al., 2014). The clade 2 nematodes (T. spiralis, T. muris) possess a near identical nlp profile, however notable differences in nlp profiles were observed within clade 8 species. Indeed, although the clade 8 filarids displayed near identical nlp profiles, they were different from that observed for A. suum which is also within clade 8. This is not surprising given that the filarid species are more closely related to each other than to A. suum, and likely reflects key differences in the lifestyles of the filarial species relative to A. suum.
3.3. Five nlps are completely conserved across key nematode species
Five Ce-nlps were completely conserved across all nine species interrogated in this study: nlp-12, -36, -47, -49 and pdf-1(nlp-74) (Table 1; Supplementary Fig. S1), highlighting their likely functional importance to nematode biology/behaviour, and supporting their presence in the last common ancestor of all nematodes. By contrast, 13 of the already annotated Ce-nlps were absent from all parasites examined (nlp-4.1, -4.2, -16, -22, -23, -39, -41, -45, -53, -65, -78, -80, and rgba-1 (nlp-84)) and so have either, been lost independently in multiple parasitic nematode lineages or, have evolved relatively recently in the specific lineage that led to C. elegans. Indeed, although all other Caenorhabditis spp. examined here possess a similar NLP profile to that of C. elegans (Supplementary Table S4), the other Caenorhabditids examined lacked nlp-39 and rgba-1/(nlp-84), suggesting that these genes arose following the split of the C. elegans lineage from C. briggsae, C. brenneri and C. remanei (~5–30 million years ago (MYA); Frézal and Félix, 2015). Similarly, the absence of nlp-4.1, -4.2, and -65 in C. japonica suggest that these genes likely arose following the separation of the ‘Japonica’ and ‘Elegans’ groups (~125–190 MYA; Lemos, 2007, Frézal and Félix, 2015). Please note that we designate the C. elegans genes F59C6.6 and F59C6.18 as nlp-4.1 and nlp-4.2, respectively, as both genes appear to have been independently annotated as ‘nlp-4′ previously (see both WormBase (WS276) and Nathoo et al., 2001).
3.4. The extent and position of NLP motif conservation is variable
The extent of the amino acid residue conservation within the NLPs encoded on each gene varied (Supplementary Fig. S1). For example, in some instances primary sequence conservation was observed across the entire length of the encoded peptide (e.g. NLP-3, -43), however in other cases conservation was biased toward either the C- (e.g. NLP-17) or N-termini (e.g. NLP-9, -21). Some NLPs (e.g. NLP-6) displayed relatively low levels of sequence conservation across the entire length of the peptide, however this did not preclude their identification using the motif-based BLAST approach employed here. These observations suggest different levels of functional constraint between peptides, and are likely indicative of the motif that is important for receptor recognition or binding.
3.5. Pan-phylum in silico analyses aid NLP prediction
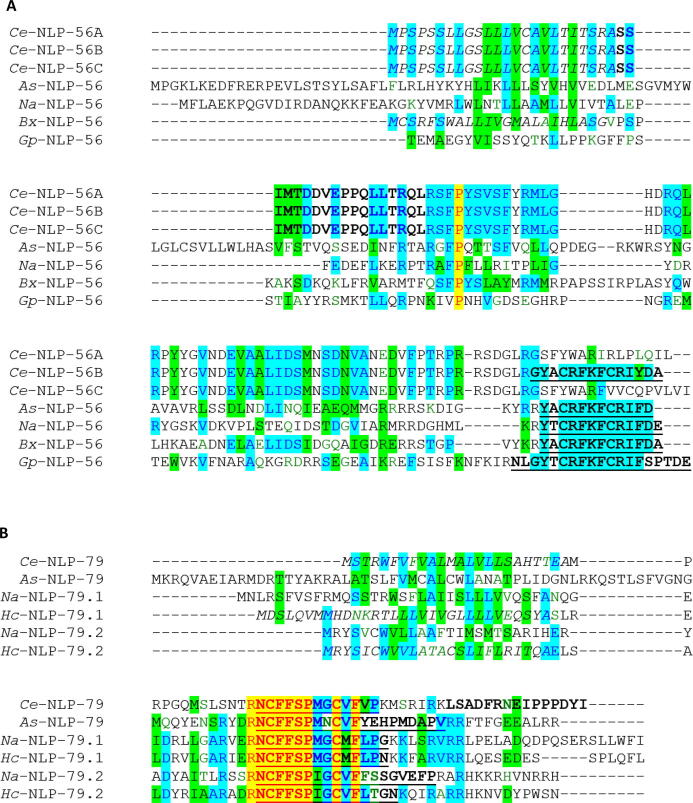
For the most part the conserved NLP motifs, identified through sequence alignments of putative prepropeptide nematode parasite NLP sequelogues, map to the previously predicted peptide regions identified via C. elegans in silico and mass spectrometry analyses (Husson et al., 2005, Van Bael et al., 2018). However, approximately one-third of the previously predicted NLP peptides, including multiple peptides identified by C. elegans mass spectrometry (Husson et al., 2005, Van Bael et al., 2018), are not conserved in any of the parasite species examined in this study (Supplementary Fig. S1; Supplementary Table S5). These peptides likely represent C. elegans-specific functionally important peptides or by-products of neuropeptide processing events. Indeed, sequence alignments of some parasite- and Ce-NLP prepropeptides (e.g. NLP-56, NLP-79; Fig. 1) reveal alternative highly conserved motifs, that were not previously considered as putative peptides (Van Bael et al., 2018), but may represent functional NLPs. These data are fundamental to the construction of the peptide libraries that seed deorphanisation efforts and underscore the value of pan-phylum in silico analyses for peptide prediction.
Fig. 1.
Novel predicted Caenorhabditis elegans neuropeptide-like proteins (Ce-NLPs) are conserved in parasitic nematodes. Multiple sequence alignments were performed using Vector NTI Advance 11.5 AlignX® (Lu and Moriyama, 2004). Text in italics highlights signal peptides as identified using Signal P 4.1 (Nielsen, 2017). Bold text indicates peptides predicted previously (see Van Bael et al., 2018). Bold underlined text indicates novel peptides predicted in this study based on their conservation in additional nematode species. Yellow and blue highlighted regions specify completely and partially conserved amino acid residues, respectively; green specifies similar residues. For all other novel NLP predictions made here (derived from nlp-8, -40, -48, -48, -52, -56, -58, -66, -67, -79 and -81 prepropeptide alignments) see Supplementary Table S5 and Supplementary Fig. S1. Ce, C. elegans; As, Ascaris suum; Na, Necator americanus; Hc, Haemonchus contortus; Bx, Bursaphelenchus xylophilius; Gp, Globodera pallida.
3.6. Parasitic nematode in silico analyses reveal novel NLP-encoding genes and highlight the need for nomenclature revision
Phylum-spanning in silico analyses of NLP-encoding genes drives novel peptide discovery in parasites and provides an opportunity for identification of additional nlps in C. elegans. This study discovered 14 novel nlps (Table 1), which have been designated nlp-85 to nlp-98. For continuity with previously identified Ce-nlps (Nathoo et al., 2001, McVeigh et al., 2008, Van Bael et al., 2018), and before naming any further novel nlp-genes identified here, we have reassigned snet-1 and rgba-1 as nlp-83 and -84, respectively. Although not classed as an nlp gene by the current version of WormBase (WS276), snet-1 (nlp-83) was identified as a C. elegans neuropeptide gene in an olfactory plasticity study and previously considered as a member of the NLP family (Yamada et al., 2010, Hobert, 2013); rgba-1 was identified as a novel C. elegans neuropeptide-encoding gene (Yin et al., 2017). The novel nlps discovered in this study include additional members of RPamides and Allatostatins, and a number of entirely novel nlps that had not previously been reported in C. elegans.
3.6.1. Novel RPamide-encoding genes were identified in parasitic nematodes
The C. elegans RPamide family consists of the nlp-2, -22 and -23 gene cluster first described by Nathoo et al. (2001), plus nlp-46, identified by McVeigh et al. (2008). The NLP-2, -22 and 23 peptides are highly similar, making sequelogue designation difficult for returned BLAST hits using the C. elegans directed BLAST approach. We therefore extended our BLAST searches using all of the RPamide encoding BLAST returns to query all available nematode genomes, and performed multiple sequence alignments to manually identify patterns of sequence conservation. Of the known RPamide encoding genes, nlp-22 and -23 sequelogues were only identified in Caenorhabditis spp., whereas nlp-2 and nlp-46 were conserved in a number of parasites (Table 1, Supplementary Table S4).
Three novel RPamide-encoding genes were identified in parasitic nematodes: (i) a novel RPamide sequence A(A/V/T)MISGRGFRPG, initially identified here in Brugia malayi and Dirofilaria immitis, and sharing a common motif with RPamides encoded in 14 other filarial species, was classified as a novel filarid-specific RPamide-encoding gene (nlp-85); (ii) a second novel RPamide peptide-encoding gene (designated here as nlp-86; GRW(G/Q)LRPG), identified initially here in A. suum, Bursaphelenchus xylophilius and Globodera pallida was also present in 11 additional nematode species representing both free-living and parasitic lifestyles; finally (iii) a third novel nlp (nlp-87; S(I/L)ALGR(F/L)(S/N)LRPG), identified initially here in A. suum, and subsequently in seven additional nematode species appears distinct from peptides encoded on nlp-2 and nlp-86.
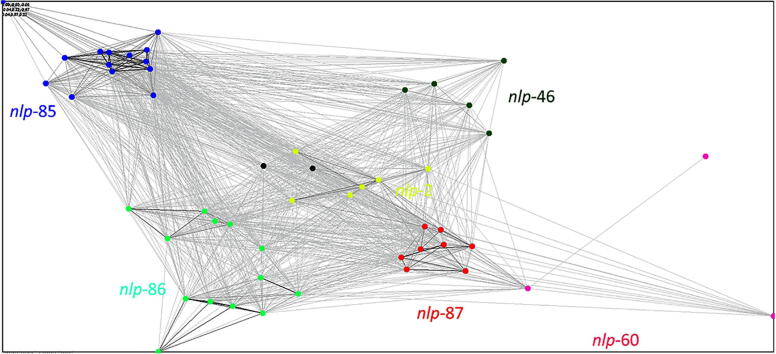
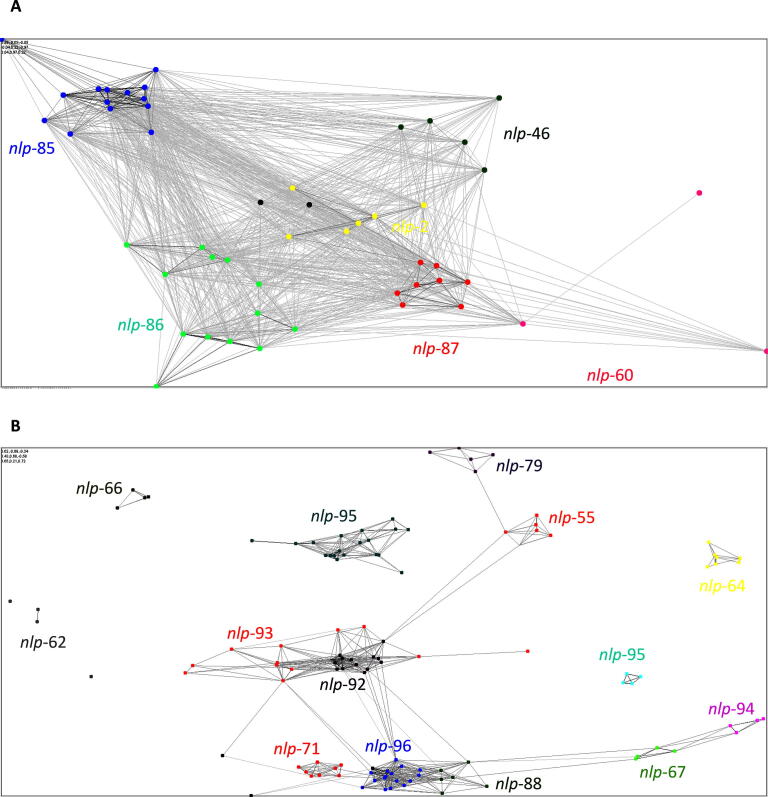
The presence of nlp-2, -86, and -87, that encode distinct RPamide peptide motifs, in the A. suum genome and subsequent CLANS analysis provides evidence to support the novel nlp designations (Fig. 2A). However, several previous studies have been unable to accurately delineate the RPamide-encoding nlps such that there are conflicting designations in the literature to what we describe here (McVeigh et al., 2008, Jarecki et al., 2011, Nelson et al., 2013, Koziol et al., 2016). This underscores the value of phylum-spanning in silico analyses to unravel the complexity of the NLP family and associated nomenclature. In addition, our approach offers a route to interrogate neuropeptide evolution; indeed, both motif and CLANS analyses reveal that nlp-60 (a non-RPamide encoding gene) likely represents an RPamide family member that is closely related to nlp-87 but has lost all RPamide peptides (Fig. 2A).
Fig. 2.
Clustered Analysis of Sequences (CLANS) clustering supports RPamide and Allatostatin-C like peptide encoding gene nomenclature. (A) Similarity matrix derived from all-against-all BLASTp comparisons between all identified nematode RPamide encoding prepropeptide sequences (E-value limit = 1). (B) Similarity matrix derived from all-against-all BLASTp comparisons between all identified nematode Allatostatin-C like prepropeptide sequences (E-value limit = 1E−5). nlp, neuropeptide-like protein.
3.6.2. Novel Allatostatin-C-like peptide encoding genes were identified in C. elegans and parasitic nematodes
Our pan-phylum motif-based and reciprocal BLAST approach enabled us to identify nine novel Allatostatin-C-like neuropeptide encoding genes (nlp-88, -89, -90, 91, -92, -93, -94, -95 and -96), in addition to those previously reported (nlp-55, -62, -64, -66, -67, -71 and -79; (Mirabeau and Joly, 2013, Van Bael et al., 2018)).
Four of the novel Allatostatin-C-like neuropeptide encoding genes (nlp-88, -89, -90 and -91) were found in the C. elegans genome, share a common RNCFF(S/T)P(V/A)QC motif, possess a signal peptide, and appear to be enriched in neurons (see WormBase (WS276): WBGene00010848 (M04B2.6); WBGene00016436 (C35B1.7); WBGene00019292 (K02A6.1); WBGene00019293 (K02A6.1)). nlp-89, -90 and -91 appear to be restricted to Caenorhabditids, whereas nlp-88 is conserved across parasitic nematodes (Table 1; Supplementary Table S4). The additional five novel Allatostatin-C-like neuropeptide encoding genes identified here (nlp-92, -93, -94, -95 and -96) share a common (K/R)NC(F/Y)F motif, are conserved across key parasite species, but are absent from C. elegans. Again, CLANS analysis broadly supports the peptide motif based novel gene designations highlighted here (Fig. 2B).
3.6.3. Novel NLP families are absent from Caenorhabditis elegans
Two additional novel putative NLP-encoding genes, that display distinct peptide motifs (NPYSW, and S(L/V)AP(T/S)TSAX3-4VS), were identified in this study and have been designated nlp-97 and -98, respectively; nlp-97 and -98 are absent from C. elegans. nlp-97 sequelogues were identified in a broad range of parasites, spanning clades 8, 9, 10 and 12 (Table 1), whilst nlp-98 appears to be Globodera spp. specific (see Supplementary Fig. S1). Note that G. pallida and Globodera rostochiensis each possess two highly similar nlp-98 sequelogues (nlp-98.1, -98.2); we also note recent nlp duplications in specific parasitic nematodes for nlp-1, -8, -10, -13, -15, -19, -21, -36, -40, -47, -61, -69, -71, -76 and -79 and have designated these paralogues as nlp-X.1, nlp-X.2 etc (see Supplementary Fig. S1).
3.7. Highly conserved NLPs are bioactive on the Ascaris suum ovijector
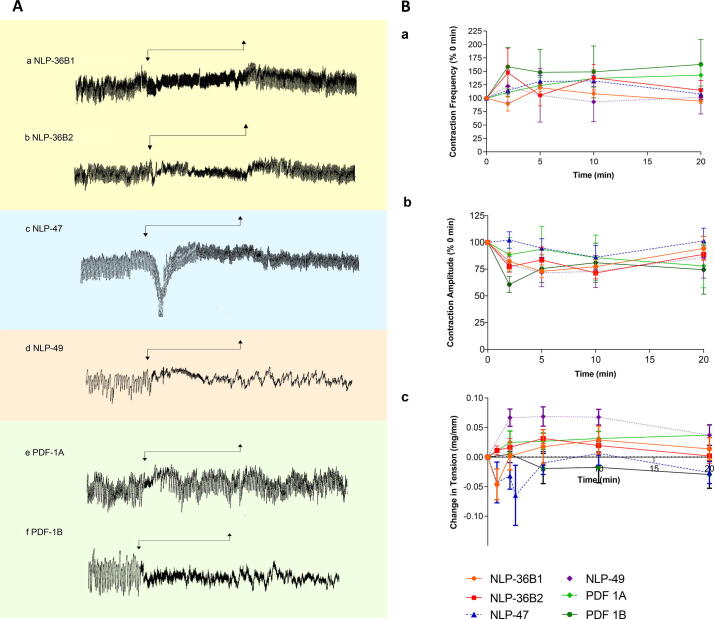
To determine the potential role of the most highly conserved NLPs in regulating muscle function, we examined the effects of the following predicted A. suum NLPs: As-PDF-1 (A and B), As-PDF-2 (C), As-NLP-12 (A1, B, C, D), NLP-36 (A1, A2, B1, B2, C1, C2), As-NLP-47 (A) and As-NLP-49 (B) (see Supplementary Table S6; Supplementary Table S7) on the reproductive musculature (ovijector) of A. suum, using established methods (Fellowes et al., 1998, Fellowes et al., 2000, Moffett et al., 2003). Note that NLP-12 (A1, B, C and D), and NLP-36 (A1, A2, C1 and C2) peptides were inactive (see Supplementary Table S6 ; Supplementary Table S7) .
NLP-36B1, -36B2, -47, PDF-1A, -1B peptides exhibited distinct excitatory effects on ovijector muscle (Fig. 3, Supplementary Table S6 ;Supplementary Table S7) as follows; (i) As-NLP-36B1 and As-NLP-36B2 induced an increase in contraction amplitude and frequency that aligned with the previously described ovijector response type (RT) 5 (Moffett et al., 2003); (ii) As-NLP-47 induced a transient lengthening of the ovijector with an increase in contraction frequency, similar to RT2 type responses, whilst (iii) As-PDF-1A and As-PDF-1B caused a decrease in contraction amplitude alongside an increase in contraction frequency that aligned to RT5. By contrast, As-NLP-49 was the only peptide tested shown to induce relaxation of the ovijector muscle (RT1; Fig. 3, Supplementary Table S6; Supplementary Table S7).
Fig. 3.
Selected neuropeptide-like protein predicted peptides (NLPs) are bioactive on the Ascaris suum ovijector muscle. (A) Representative muscle tension recordings showing the effect of As-NLP-36, As-NLP-47, As-NLP-49, and As-PDF-1 peptides. Arrows indicate peptide exposure. (B) The effects of NLPs on (i) A. suum ovijector contraction frequency, (ii) contraction amplitude, and (iii) baseline tension. Peptide exposure: 0–10 min. Scale: horizontal bar represents 1 min, vertical bar represents 1 mg.
The muscle physiology described here represents the first known reports of NLP activity on the A. suum ovijector (Moffett et al., 2003, Mousley et al., 2004, Mousley et al., 2005, McVeigh et al., 2006b, McVeigh et al., 2008). Intriguingly, pdf-1, nlp-47 and nlp-49 are all linked to the control of egg-laying in C. elegans (Lindemans et al., 2009, Meelkop et al., 2012, Chew et al., 2018), a role which may be conserved in parasitic nematodes.
4. Conclusions
This study provides a comprehensive library of NLPs in nine key parasitic nematodes, and highlights that parasites possess a reduced and variable complement of the C. elegans NLP profile. We identify 14 novel nlps, 10 of which are not found in C. elegans, bringing the total number of nlps in nematodes to 99. Five nlps display complete conservation across the nine phylogenetically dispersed parasitic nematodes examined here; four of these encode peptides that modulate nematode muscle function.
Whilst this study has been successful in the identification of >300 nlp genes across key parasitic nematode species, there are caveats associated with BLAST-based identification of neuropeptide sequelogues. Indeed, the use of C. elegans NLP sequences as BLAST queries may not uncover all parasite sequelogues, particularly in the case of those NLPs which display divergent sequences. Enhancements in genome quality for any of these species may reveal additional neuropeptide sequelogues. Note that the non-identification of a specific gene using this BLAST-based approach does not definitively prove its absence from any given parasite. The value of peptidomics approaches, particularly de novo sequencing, to neuropeptide discovery is clear when we consider that many of the more recently identified C. elegans neuropeptides are not traditionally ‘nlp-/flp-like’ (Nathoo et al., 2001, Van Bael et al., 2018). Caenorhabditis elegans data show that highly conserved neuropeptides tend to be discovered first by in silico studies, however de novo peptidomics approaches have obvious advantages when sequences are divergent from search string motifs. Nevertheless, in silico studies are highly valuable as they support exploitation of the huge amount of available in silico data to expand our knowledge of invertebrate neuropeptide signalling.
The data presented here advance our understanding of neuropeptide signalling in parasitic nematodes, support the possibility for conservation of neuropeptide function across multiple nematode species, and inform neuropeptide-receptor deorphanisation studies in therapeutically relevant parasite species.
Acknowledgements
The authors are grateful to Karro, Cookstown, NI, UK, for assistance in the collection of nematodes, and wish to thank Dr Isabel Beets and Professor Timothy G. Geary for helpful discussions. The authors acknowledge support for this work from: Biotechnology and Biological Sciences Research Council, UK, grant (BB/H019472/1), Biotechnology and Biological Sciences Research Council, UK/Boehringer Ingelheim, Germany, grants (BB/M010392/1, BB/T016396/1), and Department for the Economy Northern Ireland (DfE).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijpara.2021.07.002.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Nematode neuropeptide-like protein (NLP) sequelogue alignments from Caenorhabditis elegans and parasitic nematodes. Multiple sequence alignments were performed using Vector NTI Advance 11.5 AlignX® (Lu and Moriyama, 2004). Italic text indicates the presence of a signal peptide as identified using Signal P 4.1 (Nielsen, 2017). Ce-NLP-56A, B and C represent isoforms of the C. elegans nlp-56 gene. Parasite sequelogues designated NLP-X.1, -X.2 etc. represent paralogues (likely recent gene duplications) of C. elegans NLPs in these species. Note: pan-phylum alignments are not an exhaustive list of all NLP-sequelogues across all nematodes. Ce, C. elegans; Ts, Trichinella spiralis; Tm, Trichuris muris; As, Ascaris suum; Bm, Brugia malayi; Di, Dirofilaria immitis; Na, Necator americanus; Hc, Haemonchus contortus; Bx, Bursaphelenchus xylophilius; Gp, Globodera pallida. Yellow and blue highlighted regions indicate completely and partially conserved amino acid residues, respectively. Green indicates similar residues. References: Lu, G., Moriyama, E.N., 2004. Vector NTI, a balanced all-in-one sequence analysis suite. Brief Bioinform, 5, 378-388. Nielsen H., 2017. Predicting Secretory Proteins with SignalP. Methods Mol Biol, 1611:59-73.
Expression Heatmaps for nematode neuropeptide-like protein (NLP) genes expressed in lifecycle stages of (A) Trichuris muris, (B) Ascaris suum, (C) Brugia malayi, (D) Dirofilaria immitis, (E) Haemonchus contortus, (F) Bursaphelenchus xylophilus, (G) Globodera pallida. (A) Expression heatmap generated from log2FPKM values of seven genes in T. muris identified from developmentally staged RNA-seq libraries and https://parasite.wormbase.org utilising the Expression program in Heatmapper (http://heatmapper.ca/expression; Babicki et al. (2016)). Average Clustering Method and Pearson’s Distance Measurement Method were used. Tissues and Life stages are represented in columns (L2, L3, Mixed Adults, Adult Female, Adult Male, Anterior, Female Rear, Male Rear). Rows indicate individual genes, denoted by the gene ID. nlp-6, nlp-12, nlp-47, nlp-58, pdf-1/nlp-74, snet-1/nlp-83 have been omitted as these genes are unannotated. (B) Expression heatmap generated from log2RPKM values of 43 genes in A. suum identified from developmentally staged RNA-seq libraries and https://parasite.wormbase.org utilising the Expression program in Heatmapper (http://heatmapper.ca/expression; Babicki et al. (2016)). Average Clustering Method and Pearson’s Distance Measurement Method were used. Samples are represented in columns (L3 and Egg, L3 Liver, L3 Lung, L4, Male Muscle, Female Muscle, Male Rear, Female Rear). Rows indicate individual genes, denoted by the gene ID. nlp-9, nlp-19, nlp-35, nlp-37, nlp-42, nlp-46, nlp-49, nlp-51, nlp-57, nlp-69, nlp-71, ntc-1/nlp-75, nlp-76, nlp-87, nlp-92, nlp-93, nlp-95 have been omitted as these genes are unannotated. (C) Expression heatmap generated from log2TPM values of 29 genes in B. malayi identified from developmentally staged RNA-seq libraries and https://parasite.wormbase.org utilising the Expression program in Heatmapper (http://heatmapper.ca/expression; Babicki et al. (2016)). Average Clustering Method and Pearson’s Distance Measurement Method were used. Life stages are represented in columns (Eggs and Embryos, Microfilariae, L3, L4, Adult Female, Adult Male). Rows indicate individual genes, denoted by the gene ID. nlp-64 and nlp-71 have been omitted as these genes are unannotated. (D) Expression heatmap generated from log2FPKM values of 18 genes in D. immitis identified from developmentally staged RNA-seq libraries and https://parasite.wormbase.org utilising the Expression program in Heatmapper [http://heatmapper.ca/expression; Babicki et al. (2016)]. Average Clustering Method and Pearson’s Distance Measurement Method were used. Life stages and tissues are represented in columns (Microfilariae, L3, L4, Adult Male, Adult Female, Female Body Wall, Uterus, Female Intestine, Female Head, Male Intestine, Male Body Wall, Testes). Rows indicate individual genes, denoted by the gene ID. nlp-12, nlp-37, pdf-1/nlp-74 and nlp-81 have been omitted as these genes are unannotated. (E) Expression heatmap generated from log2FPKM values of 57 genes in H. contortus identified from developmentally staged RNA-seq libraries and https://parasite.wormbase.org utilising the Expression program in Heatmapper (http://heatmapper.ca/expression; Babicki et al. (2016)). Average Clustering Method and Pearson’s Distance Measurement Method were used. Samples are represented in columns (Egg, L1, L3, L4, Adult Female, Adult Male, Adult Male Gut). Rows indicate individual genes, denoted by the gene ID. nlp-9, nlp-11, nlp-47, nlp-50, nlp-57, nlp-59, nlp-60, nlp-65, nlp-71, pdf-1/nlp-74, ntc-1/nlp-75, nlp-81 and nlp-92 have been omitted as these genes are unannotated. nlp-18 has been omitted as no expression data is available. (F) Expression heatmap generated from log2FPKM values of 48 genes in B. xylophilus identified from developmentally staged RNA-seq libraries and https://parasite.wormbase.org utilising the Expression program in Heatmapper (http://heatmapper.ca/expression; Babicki et al. (2016)). Average Clustering Method and Pearson’s Distance Measurement Method were used. Life stages are represented in columns (L2+3h, L2+6h, L2 mixed, Stage 3 Dispersal Juvenile, L3 mixed, L4 mixed, Adult Female, Adult Male, Dauer, Mixed Embryo, Mixed Propagative). Rows indicate individual genes, denoted by the gene ID. nlp-69 has been omitted as this gene is unannotated. (G) Expression heatmap generated from log2TPM values of 38 genes in G. pallida identified from developmentally staged RNA-seq libraries and https://parasite.wormbase.org utilising the Expression program in Heatmapper (http://heatmapper.ca/expression; Babicki et al. (2016)). Average Clustering Method and Pearson’s Distance Measurement Method were used. Life stages are represented in columns (Egg, J2, 7 days p.i. (d.p.i.), 14 days p.i., 21 days p.i., 28 days p.i., 35 days p.i., Adult Male). Rows indicate individual genes, denoted by the gene ID. nlp-15, nlp-19, nlp-56, nlp-69, nlp-72 have been omitted as these genes are unannotated.
References
- Anderson R.C., Newton C.L., Millar R.P., Katz A.A. The Brugia malayi neuropeptide receptor-4 is activated by FMRFamide-like peptides and signals via Gαi. Mol. Biochem. Parasitol. 2014;195:54–58. doi: 10.1016/j.molbiopara.2014.07.002. [DOI] [PubMed] [Google Scholar]
- Atkinson L.E., Stevenson M., McCoy C.J., Marks N.J., Fleming C., Zamanian M., Day T.A., Kimber M.J., Maule A.G., Mousley A. flp-32 Ligand/receptor silencing phenocopy faster plant pathogenic nematodes. PLoS Pathog. 2013;9 doi: 10.1371/journal.ppat.1003169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger A.M., Lohse M., Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzy A., Allain C., Harrington J., Lesuisse D., Mikol V., Bruhn D.F., Maule A.G., Guillemot J.C. Peptidomics of Haemonchus contortus. ACS Omega. 2021;6:10288–10305. doi: 10.1021/acsomega.1c00650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlier J., van der Voort M., Kenyon F., Skuce P., Vercruysse J. Chasing helminths and their economic impact on farmed ruminants. Trends Parasitol. 2014;30:361–367. doi: 10.1016/j.pt.2014.04.009. [DOI] [PubMed] [Google Scholar]
- Chew Y.L., Grundy L.J., Brown A.E., Beets I., Schafer W.R. Neuropeptides encoded by nlp-49 modulate locomotion, arousal and egg-laying behaviours in Caenorhabditis elegans via the receptor SEB-3. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018;373:20170368. doi: 10.1098/rstb.2017.0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne D.L., Cortada L., Dalzell J.J., Claudius-Cole A.O., Haukeland S., Luambano N., Talwana H. Plant-parasitic nematodes and food security in Sub-Saharan Africa. Annu. Rev. Phytopathol. 2018;56:381–403. doi: 10.1146/annurev-phyto-080417-045833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierking K., Yang W., Schulenburg H. Antimicrobial effectors in the nematode Caenorhabditis elegans: an outgroup to the Arthropoda. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016;371:20150299. doi: 10.1098/rstb.2015.0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elling A.A. Major emerging problems with minor Meloidogyne species. Phytopathology. 2013;103:1092–1102. doi: 10.1094/PHYTO-01-13-0019-RVW. [DOI] [PubMed] [Google Scholar]
- Fellowes R., Maule A., Marks N., Geary T., Thompson D., Halton D. Nematode neuropeptide modulation of the vagina vera of Ascaris suum: in vitro effects of PF1, PF2, PF4, AF3 and AF4. Parasitology. 2000;120:79–89. doi: 10.1017/s0031182099005260. [DOI] [PubMed] [Google Scholar]
- Fellowes R., Maule A., Marks N., Geary T., Thompson D., Shaw C., Halton D. Modulation of the motility of the vagina vera of Ascaris suum in vitro by FMRFamide-related peptides. Parasitology. 1998;116:277–287. doi: 10.1017/s0031182097002229. [DOI] [PubMed] [Google Scholar]
- Fetterer R.H., Pax R.A., Bennett J.L. Schistosoma mansoni: Direct method for simultaneous recording of electrical and motor activity. Exp. Parasitol. 1977;43:286–294. doi: 10.1016/0014-4894(77)90033-9. [DOI] [PubMed] [Google Scholar]
- Frézal L., Félix M.A. C. elegans outside the Petri dish. Elife. 2015;4 doi: 10.7554/eLife.05849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frickey T., Lupas A. CLANS: a Java application for visualizing protein families based on pairwise similarity. Bioinformatics. 2004;20:3702–3704. doi: 10.1093/bioinformatics/bth444. [DOI] [PubMed] [Google Scholar]
- Hobert, O., 2013. The neuronal genome of Caenorhabditis elegans, WormBook, ed. The C. elegans Research Community, WormBook, doi/10.1895/wormbook.1.161.1, http://www.wormbook.org. [DOI] [PMC free article] [PubMed]
- Holterman M., van der Wurff A., van den Elsen S., van Megen H., Bongers T., Holovachov O., Bakker J., Helder J. Phylum-wide analysis of SSU rDNA reveals deep phylogenetic relationships among nematodes and accelerated evolution toward crown Clades. Mol. Biol. Evol. 2006;23:1792–1800. doi: 10.1093/molbev/msl044. [DOI] [PubMed] [Google Scholar]
- Hotez P.J., Brindley P.J., Bethony J.M., King C.H., Pearce E.J., Jacobson J. Helminth infections: the great neglected tropical diseases. J. Clin. Investig. 2008;118:1311–1321. doi: 10.1172/JCI34261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotez, P.J., Lo, N.C., 2020. Neglected tropical diseases: public health control programs and mass drug administration. In: Edward T. Ryan, David R. Hill, Tom Solomon, Naomi E. Aronson, Timothy P. Endy, (Eds.), Hunter's Tropical Medicine and Emerging Infectious Diseases (Tenth Edition). Elsevier, online, pp. 209–213.
- Howe K.L., Bolt B.J., Shafie M., Kersey P., Berriman M. WormBase ParaSite – a comprehensive resource for helminth genomics. Mol. Biochem. Parasitol. 2017;215:2–10. doi: 10.1016/j.molbiopara.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husson S.J., Clynen E., Baggerman G., De Loof A., Schoofs L. Discovering neuropeptides in Caenorhabditis elegans by two dimensional liquid chromatography and mass spectrometry. Biochem. Biophys. Res. Commun. 2005;335:76–86. doi: 10.1016/j.bbrc.2005.07.044. [DOI] [PubMed] [Google Scholar]
- Husson S.J., Mertens I., Janssen T., Lindemans M., Schoofs L. Neuropeptidergic signaling in the nematode Caenorhabditis elegans. Prog. Neurobiol. 2007;82:33–55. doi: 10.1016/j.pneurobio.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Husson S.J., Reumer A., Temmerman L., De Haes W., Schoofs L., Mertens I., Baggerman G. Worm peptidomics. EuPA Open Proteom. 2014;3:280–290. [Google Scholar]
- Jarecki J.L., Frey B.L., Smith L.M., Stretton A.O. Discovery of neuropeptides in the nematode Ascaris suum by database mining and tandem mass spectrometry. J. Proteome Res. 2011;10:3098–3106. doi: 10.1021/pr2001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan R.M., Vidyashankar A.N. An inconvenient truth: global worming and anthelmintic resistance. Vet. Parasitol. 2012;186:70–78. doi: 10.1016/j.vetpar.2011.11.048. [DOI] [PubMed] [Google Scholar]
- Kim D., Langmead B., Salzberg S.L. HISAT: a fast spliced aligner with low memory requirements. Nat. Methods. 2015;12:357–360. doi: 10.1038/nmeth.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura K.D., Tissenbaum H.A., Liu Y., Ruvkun G. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science. 1997;277:942–946. doi: 10.1126/science.277.5328.942. [DOI] [PubMed] [Google Scholar]
- Koziol U., Koziol M., Preza M., Costábile A., Brehm K., Castillo E. De novo discovery of neuropeptides in the genomes of parasitic flatworms using a novel comparative approach. Int. J. Parasitol. 2016;46:709–721. doi: 10.1016/j.ijpara.2016.05.007. [DOI] [PubMed] [Google Scholar]
- Leinonen, R., Sugawara, H., Shumway, M., International Nucleotide Sequence Database Collaboration, 2011. The sequence read archive. Nucleic Acids Res, 39, D19–D21. [DOI] [PMC free article] [PubMed]
- Lemos B. The Opossum genome reveals further evidence for regulatory evolution in mammalian diversification. Genome Biol. 2007;8:223. doi: 10.1186/gb-2007-8-8-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, C., Kim, K., 2008. Neuropeptides. WormBook, ed. The C. elegans Research Community, WormBook, doi/10.1895/wormbook.1.142.1, http://www.wormbook.org.
- Liao Y., Smyth G.K., Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30:923–930. doi: 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- Lindemans M., Liu F., Janssen T., Husson S.J., Mertens I., Gäde G., Schoofs L. Adipokinetic hormone signaling through the gonadotropin-releasing hormone receptor modulates egg-laying in Caenorhabditis elegans. Proc. Natl. Acad. Sci. U S A. 2009;106:1642–1647. doi: 10.1073/pnas.0809881106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes-Caitar V., Pinheiro J., Marcelino-Guimarães F. Nematodes in horticulture: an overview. J. Hortic. Sci. Crop. Res. 2019;1:105. [Google Scholar]
- Lu G., Moriyama E.N. Vector NTI, a balanced all-in-one sequence analysis suite. Brief Bioinform. 2004;5:378–388. doi: 10.1093/bib/5.4.378. [DOI] [PubMed] [Google Scholar]
- Marks N., Johnson S., Maule A., Halton D., Shaw C., Geary T., Moore S., Thompson D. Physiological effects of platyhelminth RFamide peptides on muscle-strip preparations of Fasciola hepatica (Trematoda: Digenea) Parasitology. 1996;113:393–401. [Google Scholar]
- Mavrot F., Hertzberg H., Torgerson P. Effect of gastro-intestinal nematode infection on sheep performance: a systematic review and meta-analysis. Parasit. Vectors. 2015;8:557. doi: 10.1186/s13071-015-1164-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy C.J., Atkinson L.E., Robb E., Marks N.J., Maule A.G., Mousley A. Tool-driven advances in neuropeptide research from a nematode parasite perspective. Trends Parasitol. 2017;33:986–1002. doi: 10.1016/j.pt.2017.08.009. [DOI] [PubMed] [Google Scholar]
- McCoy C.J., Atkinson L.E., Zamanian M., McVeigh P., Day T.A., Kimber M.J., Marks N.J., Maule A.G., Mousley A. New insights into the FLPergic complements of parasitic nematodes: Informing deorphanisation approaches. EuPA Open Proteom. 2014;3:262–272. doi: 10.1016/j.euprot.2014.04.002. https://www.frontiersin.org/articles/10.3389/fendo.2021.718363/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVeigh P., Alexander-Bowman S., Veal E., Mousley A., Marks N.J., Maule A.G. Neuropeptide-like protein diversity in phylum Nematoda. Int. J. Parasitol. 2008;38:1493–1503. doi: 10.1016/j.ijpara.2008.05.006. [DOI] [PubMed] [Google Scholar]
- McVeigh P., Atkinson L., Marks N.J., Mousley A., Dalzell J.J., Sluder A., Hammerland L., Maule A.G. Parasite neuropeptide biology: seeding rational drug target selection? Int. J. Parasitol. Drugs Drug Resist. 2012;2:76–91. doi: 10.1016/j.ijpddr.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVeigh P., Geary T.G., Marks N.J., Maule A.G. The FLP-side of nematodes. Trends Parasitol. 2006;22:385–396. doi: 10.1016/j.pt.2006.06.010. [DOI] [PubMed] [Google Scholar]
- McVeigh P., Leech S., Marks N.J., Geary T.G., Maule A.G. Gene expression and pharmacology of nematode NLP-12 neuropeptides. Int. J. Parasitol. 2006;36:633–640. doi: 10.1016/j.ijpara.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Meelkop E., Temmerman L., Janssen T., Suetens N., Beets I., Van Rompay L., Shanmugam N., Husson S.J., Schoofs L. PDF receptor signaling in Caenorhabditis elegans modulates locomotion and egg-laying. Mol. Cell. Endocrinol. 2012;361:232–240. doi: 10.1016/j.mce.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Mirabeau O., Joly J.-S. Molecular evolution of peptidergic signaling systems in bilaterians. Proc. Natl. Acad. Sci. USA. 2013;110:E2028–E2037. doi: 10.1073/pnas.1219956110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffett C., Beckett A., Mousley A., Geary T., Marks N., Halton D., Thompson D., Maule A. The ovijector of Ascaris suum: multiple response types revealed by Caenorhabditis elegans FMRFamide-related peptides. Int. J. Parasitol. 2003;33:859–876. doi: 10.1016/s0020-7519(03)00109-7. [DOI] [PubMed] [Google Scholar]
- Mousley A., Marks N.J., Halton D.W., Geary T.G., Thompson D.P., Maule A.G. Arthropod FMRFamide-related peptides modulate muscle activity in helminths. Int. J. Parasitol. 2004;34:755–768. doi: 10.1016/j.ijpara.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Mousley A., Moffett C.L., Duve H., Thorpe A., Halton D.W., Geary T.G., Thompson D.P., Maule A.G., Marks N.J. Expression and bioactivity of allatostatin-like neuropeptides in helminths. Int. J. Parasitol. 2005;35:1557–1567. doi: 10.1016/j.ijpara.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Nathoo A.N., Moeller R.A., Westlund B.A., Hart A.C. Identification of neuropeptide-like protein gene families in Caenorhabditis elegans and other species. Proc. Natl. Acad. Sci. USA. 2001;98:14000–14005. doi: 10.1073/pnas.241231298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson M.D., Trojanowski N.F., George-Raizen J.B., Smith C.J., Yu C.C., Fang-Yen C., Raizen D.M. The neuropeptide NLP-22 regulates a sleep-like state in Caenorhabditis elegans. Nat. Commun. 2013;4:2846. doi: 10.1038/ncomms3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen H. Predicting secretory proteins with SignalP. Methods Mol. Biol. 2017;1611:59–73. doi: 10.1007/978-1-4939-7015-5_6. [DOI] [PubMed] [Google Scholar]
- Payne L., Fitchett J.R. Bringing neglected tropical diseases into the spotlight. Trends Parasitol. 2010;26:421–423. doi: 10.1016/j.pt.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Pujol N., Zugasti O., Wong D., Couillault C., Kurz C.L., Schulenburg H., Ewbank J.J. Anti-fungal innate immunity in C. elegans is enhanced by evolutionary diversification of antimicrobial peptides. PLoS Pathog. 2008;4 doi: 10.1371/journal.ppat.1000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeber F., Jex A.R., Gasser R.B. Impact of gastrointestinal parasitic nematodes of sheep, and the role of advanced molecular tools for exploring epidemiology and drug resistance-an Australian perspective. Parasit. Vectors. 2013;6:153. doi: 10.1186/1756-3305-6-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangster N.C., Cowling A., Woodgate R.G. Ten events that defined anthelmintic resistance research. Trends Parasitol. 2018;34:553–563. doi: 10.1016/j.pt.2018.05.001. [DOI] [PubMed] [Google Scholar]
- Van Bael S., Zels S., Boonen K., Beets I., Schoofs L., Temmerman L. A Caenorhabditis elegans mass spectrometric resource for neuropeptidomics. J. Am. Soc. Mass Spectrom. 2018;29:879–889. doi: 10.1007/s13361-017-1856-z. [DOI] [PubMed] [Google Scholar]
- Warnock N.D., Wilson L., Patten C., Fleming C.C., Maule A.G., Dalzell J.J. Nematode neuropeptides as transgenic nematicides. PLoS Pathog. 2017;13:e1006237. doi: 10.1371/journal.ppat.1006237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K., Hirotsu T., Matsuki M., Butcher R.A., Tomioka M., Ishihara T., Clardy J., Kunitomo H., Iino Y. Olfactory plasticity is regulated by pheromonal signaling in Caenorhabditis elegans. Science. 2010;329:1647–1650. doi: 10.1126/science.1192020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J.A., Gao G., Liu X.J., Hao Z.Q., Li K., Kang X.L., Li H., Shan Y.H., Hu W.L., Li H.P., Cai S.Q. Genetic variation in glia-neuron signalling modulates ageing rate. Nature. 2017;551:198–203. doi: 10.1038/nature24463. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Nematode neuropeptide-like protein (NLP) sequelogue alignments from Caenorhabditis elegans and parasitic nematodes. Multiple sequence alignments were performed using Vector NTI Advance 11.5 AlignX® (Lu and Moriyama, 2004). Italic text indicates the presence of a signal peptide as identified using Signal P 4.1 (Nielsen, 2017). Ce-NLP-56A, B and C represent isoforms of the C. elegans nlp-56 gene. Parasite sequelogues designated NLP-X.1, -X.2 etc. represent paralogues (likely recent gene duplications) of C. elegans NLPs in these species. Note: pan-phylum alignments are not an exhaustive list of all NLP-sequelogues across all nematodes. Ce, C. elegans; Ts, Trichinella spiralis; Tm, Trichuris muris; As, Ascaris suum; Bm, Brugia malayi; Di, Dirofilaria immitis; Na, Necator americanus; Hc, Haemonchus contortus; Bx, Bursaphelenchus xylophilius; Gp, Globodera pallida. Yellow and blue highlighted regions indicate completely and partially conserved amino acid residues, respectively. Green indicates similar residues. References: Lu, G., Moriyama, E.N., 2004. Vector NTI, a balanced all-in-one sequence analysis suite. Brief Bioinform, 5, 378-388. Nielsen H., 2017. Predicting Secretory Proteins with SignalP. Methods Mol Biol, 1611:59-73.
Expression Heatmaps for nematode neuropeptide-like protein (NLP) genes expressed in lifecycle stages of (A) Trichuris muris, (B) Ascaris suum, (C) Brugia malayi, (D) Dirofilaria immitis, (E) Haemonchus contortus, (F) Bursaphelenchus xylophilus, (G) Globodera pallida. (A) Expression heatmap generated from log2FPKM values of seven genes in T. muris identified from developmentally staged RNA-seq libraries and https://parasite.wormbase.org utilising the Expression program in Heatmapper (http://heatmapper.ca/expression; Babicki et al. (2016)). Average Clustering Method and Pearson’s Distance Measurement Method were used. Tissues and Life stages are represented in columns (L2, L3, Mixed Adults, Adult Female, Adult Male, Anterior, Female Rear, Male Rear). Rows indicate individual genes, denoted by the gene ID. nlp-6, nlp-12, nlp-47, nlp-58, pdf-1/nlp-74, snet-1/nlp-83 have been omitted as these genes are unannotated. (B) Expression heatmap generated from log2RPKM values of 43 genes in A. suum identified from developmentally staged RNA-seq libraries and https://parasite.wormbase.org utilising the Expression program in Heatmapper (http://heatmapper.ca/expression; Babicki et al. (2016)). Average Clustering Method and Pearson’s Distance Measurement Method were used. Samples are represented in columns (L3 and Egg, L3 Liver, L3 Lung, L4, Male Muscle, Female Muscle, Male Rear, Female Rear). Rows indicate individual genes, denoted by the gene ID. nlp-9, nlp-19, nlp-35, nlp-37, nlp-42, nlp-46, nlp-49, nlp-51, nlp-57, nlp-69, nlp-71, ntc-1/nlp-75, nlp-76, nlp-87, nlp-92, nlp-93, nlp-95 have been omitted as these genes are unannotated. (C) Expression heatmap generated from log2TPM values of 29 genes in B. malayi identified from developmentally staged RNA-seq libraries and https://parasite.wormbase.org utilising the Expression program in Heatmapper (http://heatmapper.ca/expression; Babicki et al. (2016)). Average Clustering Method and Pearson’s Distance Measurement Method were used. Life stages are represented in columns (Eggs and Embryos, Microfilariae, L3, L4, Adult Female, Adult Male). Rows indicate individual genes, denoted by the gene ID. nlp-64 and nlp-71 have been omitted as these genes are unannotated. (D) Expression heatmap generated from log2FPKM values of 18 genes in D. immitis identified from developmentally staged RNA-seq libraries and https://parasite.wormbase.org utilising the Expression program in Heatmapper [http://heatmapper.ca/expression; Babicki et al. (2016)]. Average Clustering Method and Pearson’s Distance Measurement Method were used. Life stages and tissues are represented in columns (Microfilariae, L3, L4, Adult Male, Adult Female, Female Body Wall, Uterus, Female Intestine, Female Head, Male Intestine, Male Body Wall, Testes). Rows indicate individual genes, denoted by the gene ID. nlp-12, nlp-37, pdf-1/nlp-74 and nlp-81 have been omitted as these genes are unannotated. (E) Expression heatmap generated from log2FPKM values of 57 genes in H. contortus identified from developmentally staged RNA-seq libraries and https://parasite.wormbase.org utilising the Expression program in Heatmapper (http://heatmapper.ca/expression; Babicki et al. (2016)). Average Clustering Method and Pearson’s Distance Measurement Method were used. Samples are represented in columns (Egg, L1, L3, L4, Adult Female, Adult Male, Adult Male Gut). Rows indicate individual genes, denoted by the gene ID. nlp-9, nlp-11, nlp-47, nlp-50, nlp-57, nlp-59, nlp-60, nlp-65, nlp-71, pdf-1/nlp-74, ntc-1/nlp-75, nlp-81 and nlp-92 have been omitted as these genes are unannotated. nlp-18 has been omitted as no expression data is available. (F) Expression heatmap generated from log2FPKM values of 48 genes in B. xylophilus identified from developmentally staged RNA-seq libraries and https://parasite.wormbase.org utilising the Expression program in Heatmapper (http://heatmapper.ca/expression; Babicki et al. (2016)). Average Clustering Method and Pearson’s Distance Measurement Method were used. Life stages are represented in columns (L2+3h, L2+6h, L2 mixed, Stage 3 Dispersal Juvenile, L3 mixed, L4 mixed, Adult Female, Adult Male, Dauer, Mixed Embryo, Mixed Propagative). Rows indicate individual genes, denoted by the gene ID. nlp-69 has been omitted as this gene is unannotated. (G) Expression heatmap generated from log2TPM values of 38 genes in G. pallida identified from developmentally staged RNA-seq libraries and https://parasite.wormbase.org utilising the Expression program in Heatmapper (http://heatmapper.ca/expression; Babicki et al. (2016)). Average Clustering Method and Pearson’s Distance Measurement Method were used. Life stages are represented in columns (Egg, J2, 7 days p.i. (d.p.i.), 14 days p.i., 21 days p.i., 28 days p.i., 35 days p.i., Adult Male). Rows indicate individual genes, denoted by the gene ID. nlp-15, nlp-19, nlp-56, nlp-69, nlp-72 have been omitted as these genes are unannotated.