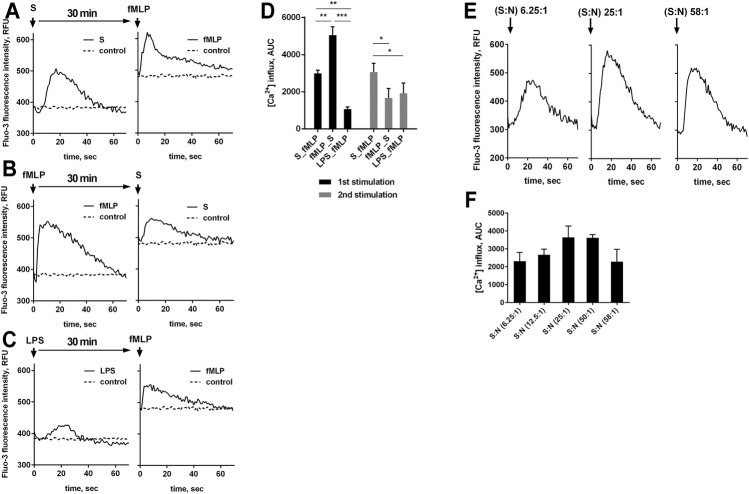
FIGURE 2.
A-D. fMLP-induced increase in [Ca2+]i in the presence of bacteria or LPS. Fluo-3 AM loaded PMNLs (4 × 105/sample) were cultured in fibrinogen-coated flat bottom 96-well plates in HBSS/HEPES medium at 37°C, 5% CO2. Cells were sequential exposed to nonopsonized S. typhimurium (S) (bacteria per cell ratio 25:1), LPS (2 μg/ml) and 0.1 µM fMLP with an interval of 30 min (the order of adding stimuli is shown in the figure). HBSS/HEPES was added to control samples (dotted lines on A-C). Flash kinetic of Fluo-3 fluorescence (ex. 488 nm, em. 535 nm) was monitored with 1 s interval. (A, B, C) Changes in [Ca2+]i are presented as typical blank corrected Fluo-3 fluorescence kinetic curves for each type of stimulation. Diagram (D) show areas under curves (AUC) with control values as baseline for fluorescence obtained over 70 s after the addition of stimuli. Values indicate mean ± SEM, n = 3, *p < 0.05, **p < 0.01, ***p < 0.001 for pairs of data compared as indicated by two-way ANOVA followed by Tukey’s multiple comparison test. (E, F). [Ca2+]i changes in neutrophils following S. typhimurium exposure at various bacterial load. Fluo-3 AM loaded PMNLs (4 × 105/sample) were cultured in fibrinogen-coated flat bottom 96-well plates in HBSS/HEPES medium at 37°C, 5% CO2. PMNLs were stimulated with nonopsonized bacteria with an increase in bacterial load from 6.25 to 58 bacteria per cell (as indicated). Flash kinetic of Fluo-3 fluorescence (ex. 488 nm, em. 535 nm) was monitored with 1 s interval. (E) Changes in [Ca2+]i are presented as typical blank corrected Fluo-3 fluorescence kinetic curves for the average (25: 1) and extreme values of the studied range of bacterial load. AUC’s for fluorescence obtained over 70 s after the addition of bacteria are represented on (F) (values indicate mean ± SEM, n = 3).