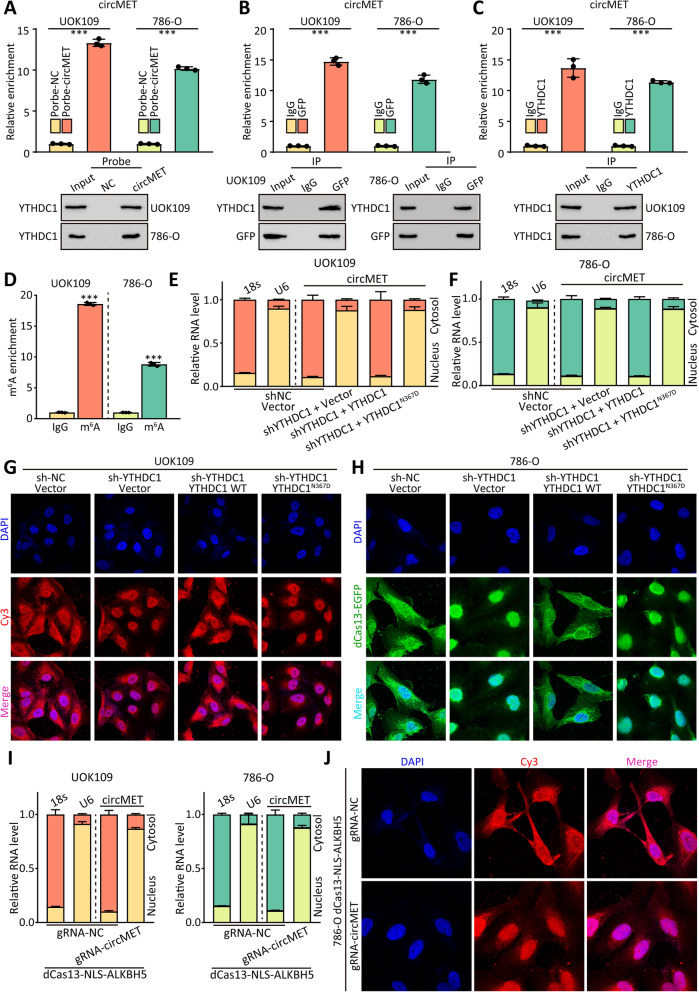
Fig. 4.
CircMET is exported to cytoplasm by YTHDC1 in m6A-depend manner. (A) The circMET-protein complex was pulled down by circMET junction probe with protein extracts from UOK109 and 786-O cells. The efficiency of circMET junction probe was detected by real-time PCR (upper), and the enrichment of YTHDC1 was detected by western blot (lower). (B-C) MS2-RIP and RIP assays were performed to confirm the association of YTHDC1 with circMET. The relative enrichment of circMET associated with YTHDC1 was detected by real-time PCR (upper), and IP efficiency of YTHDC1-antibody was showed in western blot (lower). IgG antibody served as a control. (D) Abundance of circMET among RNA immunoprecipitated with anti-m6A antibody in UOK109 and 786-O cells was measured by real-time PCR and normalized to IgG. (E–F) The subcellular distribution of circMET was analyzed via real-time PCR in UOK109 and 786-O cells after transfected with indicated lentivirus. U6 and 18s rRNA were used as nuclear and cytoplasmic markers, respectively. (G) The location of circMET (red) in UOK109 transfected with indicated lentivirus was determined by FISH assay. DAPI-stained nuclei are blue. (H) CircMET (green) in UOK109 transfected with indicated lentivirus was lighted by dCas13-3 × EGFP and gRNA targeted to circMET. DAPI-stained nuclei are blue. (I) The subcellular distribution of circMET was analyzed via real-time PCR in UOK109 and 786-O cells after transfected with indicated lentivirus. U6 and 18s rRNA were used as nuclear and cytoplasmic markers, respectively. (J) The location of circMET (red) in UOK109 transfected with indicated lentivirus was determined by FISH assay. DAPI-stained nuclei are blue. The data are presented as the mean ± SD, ***P < 0.001