Figure 5. 15-LOX-1 Decreases Cell Membranous LRP5 Levels.
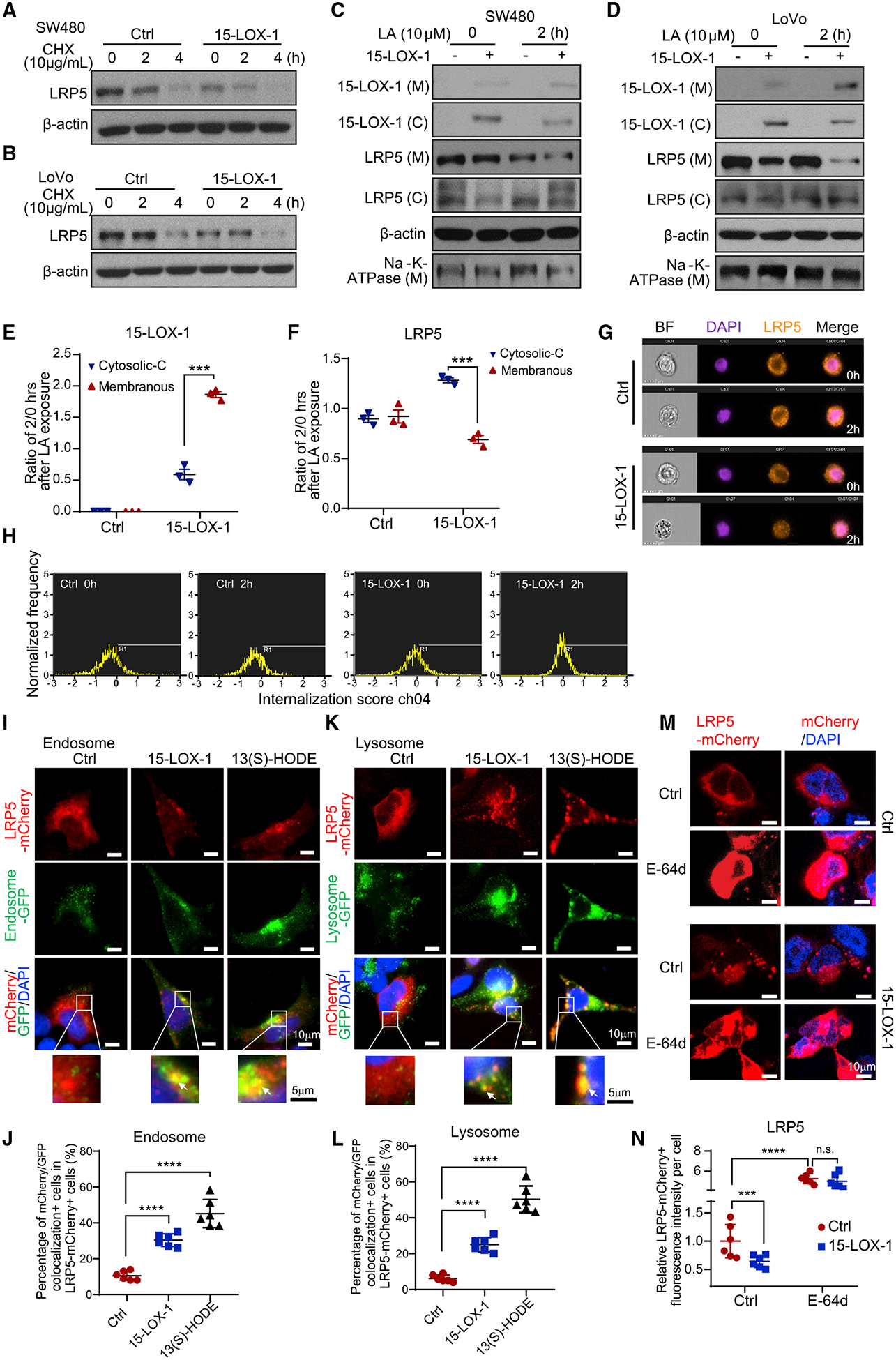
(A and B) LRP5 protein expression in SW480 (A) and LoVo (B) cells stably transduced with control (Ctrl) or 15-LOX-1 lentivirus and treated with 10 μg/mL cycloheximide (CHX) for 0, 2, and 4 h. The whole-cell lysates were analyzed using western blot.
(C and D) Cytoplasmic (C) and membranous (M) LRP5 and 15-LOX-1 protein expression in SW480 (C) and LoVo (D) cells stably transduced with control (Ctrl) or 15-LOX-1 lentivirus and treated with 10 μM linoleic acid (LA) for 2 h. The cells were processed into cytoplasmic and membranous protein fractions and then analyzed using western blot.
(E and F) Two hour to 0 h ratios of cytoplasmic protein band densities normalized to β-actin and ratios of membranous protein band densities normalized to Na+-K+-ATPase for 15-LOX-1 (E) and LRP5 (F) expression in SW480 cells transduced with Ctrl or 15-LOX-1 lentivirus, corresponding to (C) (n = 3). Values are mean ± SEM. ***p < 0.001 (two-way ANOVA).
(G and H) SW480 cells stably transduced with Ctrl or 15-LOX-1 lentivirus were treated with 10 μM LA for 2 h. The cells were analyzed for LRP5 internalization using imaging flow cytometry assay. Representative images (G) and cell internalization distribution (H) of 5,000 examined cells (cells with internalization score R1 > 0 were counted as internalization positive) are shown (n = 3 repeats with similar results).
(I–L) 293T cells stably transfected with Tet-on 15-LOX-1 inducible vector were transfected with mCherry-tagged LRP5 expression vector and treated with (15-LOX-1) or without (Ctrl) doxycycline (2 μg/mL) or treated with 13(S)-HODE (27 μM) for 24 h and then incubated with CellLight Early Endosomes-GFP, BacMam 2.0 or CellLight Lysosomes-GFP, BacMam 2.0 for another 24 h. Representative microphotographs and quantitative results of mCherry-labeled LRP5 colocalization with GFP-traced cytoplasmic endosomes (I and J) or lysosomes (K and L) are shown. Quantification was evaluated as percentages of the cells that had co-localization of mCherry and GFP fluorescence out of mCherry-positive cells from six random fields under a confocal microscope. Arrows indicate colocalization. (M and N) 293T cells were co-transfected with mCherry-tagged LRP5 expression plasmid with pCMV5-IRES-GFP (Ctrl) or with pCMV5–15-LOX-1-IRES-GFP (15-LOX-1) for 48 h and then treated with E-64d (10 μM) or dissolvent (Ctrl) for 1 h. Representative microphotographs (M) and quantitative results (N) of LRP5 trans-localization traced with mCherry fluorescence protein from six random fields under a confocal microscope are shown.
Values are mean ± SD. ***p < 0.001, ****p < 0.0001, n.s., no significant difference; one-way ANOVA (J and L), two-way ANOVA (N). n = 3 repeats with similar results.
