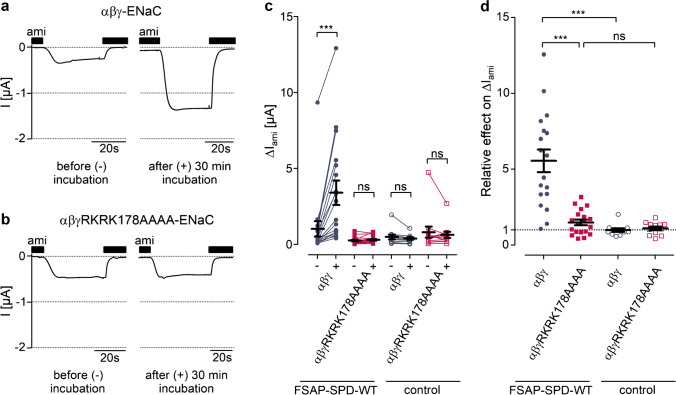
Fig. 3.
Mutation of ENaC at its putative prostasin cleavage site prevents its proteolytic activation by recombinant serine protease domain of FSAP. a, b Representative whole-cell current traces recorded in oocytes expressing human wild-type ENaC (αβγ-ENaC; a) or coexpressing wild-type α- and β-ENaC with mutant γ-ENaC (αβγRKRK178AAAA-ENaC; b) before (left panels) and after (right panels) 30 min incubation in a solution containing FSAP-SPD-WT (20 µg mL−1). c Summary of data obtained from similar experiments as shown in a, b and from additional experiments in which protease-free ND96 was used as control. ΔIami was determined before ( −) and after ( +) incubation in the indicated incubation solution. Measurements performed in the same oocyte are connected by a line (N = 2–3, n = 11–19). d Summary of the same data as shown in c normalized as relative effect of the indicated incubation solution on ΔIami (N = 2–3, n = 11–19). ‡p < 0.001; ns non-significant; paired t-test (c) or one-way ANOVA with Bonferroni post hoc test (d). Error bars, S.E. N indicates the number of different batches of oocytes; n indicates the numbers of individual oocytes measured