Table 1.
Optimized conditions for the reactiona.
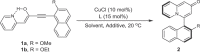 | |||||||
|---|---|---|---|---|---|---|---|
| Entry | Catalyst | Ligand | Solvent | Additives | t (h) | Yield (%)b | Ee (%)c |
| 1 | AgOTf | L1 | DCE | – | 0.5 | 70 | 29 |
| 2 | CuBF4 | L1 | DCE | – | 72 | 50 | 36 |
| 3 | CuCl | L1 | DCE | – | 72 | 51 | 73 |
| 4 | CuCl | L1 | THF | – | 72 | 38 | 69 |
| 5 | CuCl | L1 | MeCN | – | 72 | 34 | 56 |
| 6 | CuCl | L1 | Toluene | – | 72 | 45 | 76 |
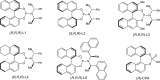
| 7 | CuCl | L2 | Toluene | – | 72 | 30 | 20 |
| 8 | CuCl | L3 | Toluene | – | 72 | 35 | 46 |
| 9 | CuCl | L4 | Toluene | – | 72 | 56 | 69 |
| 10 | CuCl | L5 | Toluene | – | 72 | 30 | 35 |
| 11d | CuCl | L1 | Toluene | – | 72 | 51 | 90 |
| 12d | CuCl | L1 | Toluene (2.0 mL) | – | 72 | 70 | 90 |
| 13d | CuCl | L1 | Toluene (3.0 mL) | – | 72 | 58 | 90 |
| 14d | CuCl | L1 | Toluene | (R)-CPA | 36 | 88 | 92 |
| 15d | CuCl | L1 | Toluene | TsOH | 36 | 67 | 90 |
| 16d | CuCl | L1 | Toluene | PhCOOH | 36 | 57 | 90 |
| 17d | CuCl | L1 | Toluene | (R)-CPA (0.5 eq) | 36 | 83 | 91 |
| 18d | CuCl | L1 | Toluene | (R)-CPA (0.2 eq) | 36 | 76 | 90 |
| 19d | CuCl | L1 | Toluene | NaBArF | 72 | 26 | 13 |
| 20d | CuCl | L1 | Toluene | DBU | 72 | N.D.e | N.D.e |
 | |||||||
aReaction conditions: 1a (0.1 mmol), catalyst (10 mol%), ligand (15 mol%), solvent (1.0 mL), 20 °C.
bYields of isolated products.
cEe values were determined by chiral HPLC.
d1b was used as the substrate.
eN.D. = Not detected. Compound 1b was decomposed.
