Abstract
Hepatic angiosarcoma is an uncommon primary malignancy of the liver. It carries a poor prognosis because of very aggressive nature of the tumor. Clinical presentation of hepatic angiosarcoma is variable, most common being hepatomegaly or abdominal mass. Here we report a case of primary hepatic angiosarcoma presented with spontaneous rupture and hemoperitoneum, which was managed successfully by transarterial embolization.
Keywords: hepatic angiosarcoma, rupture, hemoperitoneum, transarterial embolization
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; aPTT, activated partial thromboplastin time; AST, aspartate aminotransferase; HBsAg, hepatitis B surface antigen; HCV, hepatitis C virus; INR, international normalized ratio; PHA, primary hepatic angiosarcoma
Hepatic malignancies of mesenchymal origin are uncommon and they constitute <1% of primary malignant liver tumors. Primary hepatic angiosarcoma (PHA) is the most common (36%) primary liver malignancy of mesenchymal origin.1 Overall, it is the third commonest primary malignancy of liver after hepatocellular carcinoma and intrahepatic cholangiocarcinoma. The disease has a male predilection.1 PHA arises from the endothelium of blood vessels. Thorotrast, arsenic, and vinyl chloride exposure are implicated in an increased risk of development of angiosarcoma.2,3 Symptoms and signs of hepatic angiosarcoma are nonspecific and include pain, fever, abdominal lump, weight loss, anemia, and hemoperitoneum. Owing to rapid progression, resistance to chemoradiation, and high rate of recurrence, hepatic angiosarcoma has a very poor prognosis and most patients die within 2 years of diagnosis.4,5 Even liver transplantation has not been described much beneficial in such patients.6 Being an aggressive vascular tumor with a strong tendency of rupture and intraperitoneal hemorrhage, emergent transarterial embolization is definitely beneficial. Here we report a case of ruptured PHA, which was treated by catheter- directed embolization.
Case report
A 69-year-old male complained about right upper quadrant pain abdomen for one and a half months. The pain was insidious in onset and gradually decrease in intensity. There was no history of any abdominal trauma, abdominal distension, hematemesis, melaena, jaundice, fever, loss of appetite, or weight. The patient was a chronic alcoholic with an alcohol intake of 120 ml/day for the last 30 years. He had a history of hypertension but was not on any regular medication. There was no history of diabetes, tuberculosis, or coronary artery disease. Physical examination revealed hepatomegaly with tenderness in right hypochondrium. The patient was conscious, oriented, and afebrile. He had elevated blood pressure (154/90 mmHg) with a normal pulse rate (88 beats/min). Blood investigations revealed normal hemogram (hemoglobin 11 g/dL, total leukocyte count 8.1 × 103/microliter, platelet 214 × 103/microliter), electrolytes (sodium 135.4 mmol/L, potassium 3.8 mmol/L, chloride 100.2 mmol/L), liver function test (total bilirubin 0.53 mg/dl, conjugated bilirubin 0.3 mg/dl, aspartate aminotransferase 28.8 U/L, alanine aminotransferase 25.1 U/L, alkaline phosphatase 62 U/L, total protein 6.7 g/dl, albumin 3.7 g/dl), coagulogram (prothrombin index 78%, international normalized ratio 1.2, activated partial thromboplastin time 28.1 s), and renal function test (urea 32.8 mg/dl, creatinine 1.2 mg/dl). Hepatitis B surface antigen and anti–hepatitis C virus antibodies were nonreactive. Alfa fetoprotein was mildly elevated (28.06 ng/ml) with a normal CA 19-9 level (4.8 U/ml).
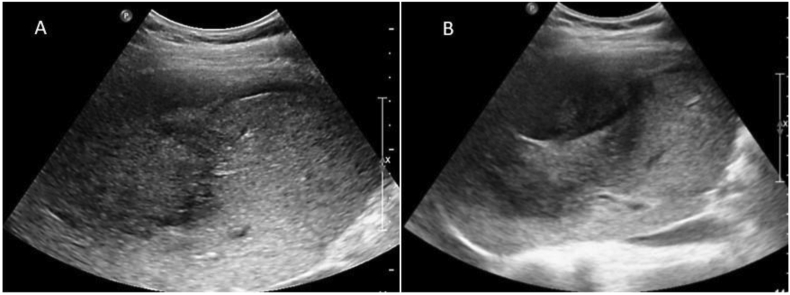
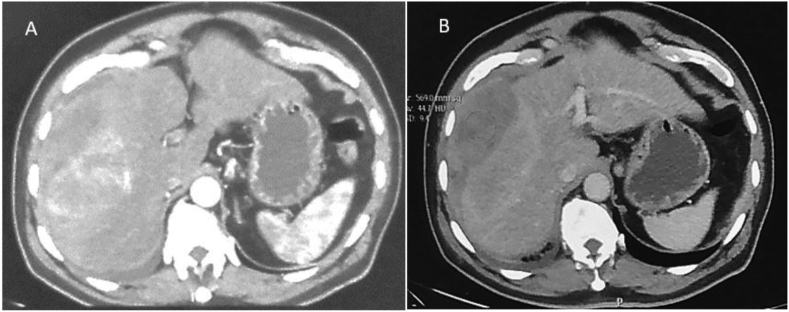
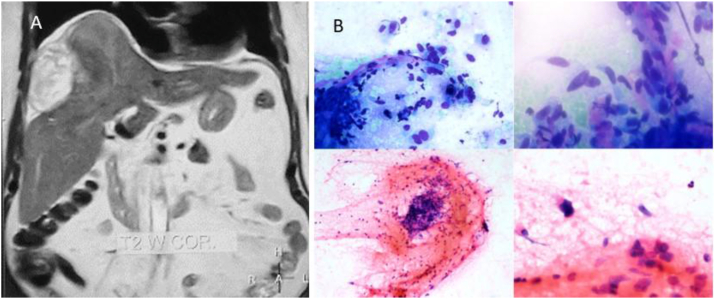
The ultrasound examination of the abdomen showed a large heteroechoic mass in the right lobe of the liver with capsular breach with perihepatic fluid (Figure 1A&B). Biphasic computed tomography and noncontrast magnetic resonance imaging showed a heterogenous mass in the right lobe, predominantly in segments V, VII, and VIII with capsular breach, perihepatic extension, and fluid suggestive of contained rupture. The arterial phase of computed tomography showed patchy nonperipheral enhancement (Figure 2A). The portovenous phase showed heterogenous enhancement of the mass with no early washout of contrast (Figure 2B). No fat or calcification was present within the mass. Hepatic and portal veins were normal. Intrahepatic biliary radicles were not dilated. Coronal T2-weighted magnetic resonance image (Figure 3A) shows a heterogeneously hyperintense mass that has ruptured with mild perihepatic fluid. Micrograph revealed clusters of tumor cells with moderately pleomorphic nuclei and fibrillary cytoplasm along with few markedly pleomorphic cells features of which were consistent with mesenchymal tumor (Figure 3B).
Figure 1.
(A & B): Grayscale ultrasonography images show heterogeneously hyperechoic right lobe mass with capsular discontinuity and rim of perihepatic fluid.
Figure 2.
(A & B): Axial section of arterial (A) and venous phase (B) of computed tomography show arterial hypervascular mass with contained rupture. Minimal pleural effusion is noted.
Figure 3.
(A & B): (A) Coronal T2WI shows a heterogeneously hyperintense mass in the right lobe. (B) Micrographs show clusters of tumor cells with moderately pleomorphic nuclei and fibrillary cytoplasm.
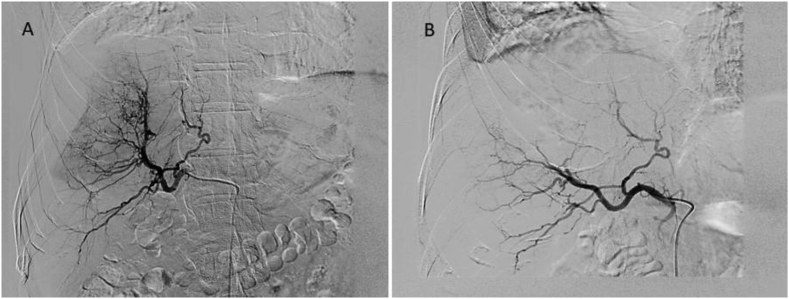
Keeping in mind the fact of a ruptured liver tumor, the patient was taken for bland embolization. Hepatic artery proper angiogram showed abnormal tumor blush in the right lobe. The feeder was seen arising from a branch of the right hepatic artery (Figure 4A). No active contrast extravasation from the tumor surface was seen. Super selective cannulation into the feeder was done using a 2.7Fr microcatheter and then embolization was done using 2 ml of embosphere 500–700 and gelfoam powder mixed with contrast material. Postembolization angiogram showed disappearance of abnormal tumor blush (Figure 4B). Postprocedure period was uneventful and the patient is being worked up for surgery.
Figure 4.
(A &B): (A) Diagnostic angiogram from common hepatic artery shows abnormal tumor blush in the right lobe with feeders from a branch of the right hepatic artery. (B) Postembolization angiogram shows complete occlusion of the feeder with no tumor blush.
Discussion
Angiosarcomas are aggressive mesenchymal malignant tumors. They occur most frequently in head and neck region, and liver is the 5th most common site of origin.7 Most commonly, it occurs in patients in the 6th to 7th decade with a male predominance (M: F = 3 to 4:1). In 75% of cases, etiology is not known, but the most common known etiologic factors are exposure to vinyl chloride, iatrogenic exposure to Thorotrast, use of androgenic steroid, and chronic arsenic ingestion. It has a prolonged latency period of 10–40 years for the development of hepatic angiosarcoma because of environmental exposure.8 Hepatic angiosarcoma may present as multiple masses or a single heterogenous mass (due to internal hemorrhage). Imaging findings are variable and usually show heterogenous enhancement with progressive filling in contrast studies. Being hypervascular in nature, it mimics other hypervascular hepatic lesions like hemangioma, adenoma, or hepatocellular carcinoma and differentiation of PHA from these lesions is difficult on imaging. Hemangioma is common in women and demonstrates discontinuous nodular peripheral enhancement in the arterial phase with progressive centripetal fill-in in portal venous and delayed phases. Hepatic adenoma usually occurs in young women on oral contraceptive or young men on anabolic steroid. On imaging, it is a well-marginated tumor in a normal background liver showing early arterial enhancement, which becomes isodense/isointense on portal venous and delayed phases. Heterogenicity may be present in the presence of intratumoral fat and hemorrhage. Hepatocellular carcinoma is an arterial hypervascular tumor showing rapid washout in the portal venous phase and typically occurs in a cirrhotic background. Many times, patients with angiosarcoma present with vague nonspecific symptoms in spite of having underlying aggressive tumor and diagnosis of angiosarcoma is made only at autopsy.9 However, in some instances, the initial presentation of angiosarcoma occurs as spontaneous rupture and life-threatening intraperitoneal bleeding.9 Massive postbiopsy bleeding in angiosarcoma has also been reported in the literature.10 Even if PHA is diagnosed antemortem, mostly they are already in an advanced stage with very limited therapeutic options with surgical resection being the cornerstone of treatment. Acute presentation with intraperitoneal bleed has a high mortality rate with a high chance of recurrent bleeding even after initial transarterial embolization of ruptured tumor. Once the tumor ruptures, transarterial embolization is the first choice of treatment to control bleeding and to stabilize the patient.11 Permanent particulate embolizing materials are the agent of choice as they penetrate the tumor and reduce its vascularity. In case of active bleed from large caliber vessels, coils can be used to stop the bleeding. After embolization and resuscitation, suitable candidates can be considered for surgical resection.
Hepatic angiosarcoma can rarely present with rupture and hemoperitoneum, which can be life-threatening requiring emergency interventions. In such a scenario, the initial management should be to stabilize the patient with catheter-directed angioembolization to stop the bleeding.
CRediT authorship contribution statement
Gaurav C. Das: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. Sreedhara B. Chaluvashetty: Conceptualization, Methodology, Writing – review & editing, Supervision. Shruti Gupta: Methodology, provision of pathology slides. Arka De: Conceptualization, Methodology.
Conflicts of interest
The authors have none to declare.
Funding
None.
References
- 1.Molina E and Hernandez A: Clinical manifestations of primary hepatic angiosarcoma. Dig Dis Sci. 48:677–682. [DOI] [PubMed]
- 2.Ito Y., Kojiro M., Nakashima T., Mori T. Pathomorphologic characteristics of 102 cases of thorotrast-related hepatocellular carcinoma, cholangiocarcinoma, and hepatic angiosarcoma. Cancer. 1988;62:1153–1162. doi: 10.1002/1097-0142(19880915)62:6<1153::aid-cncr2820620619>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 3.Makk L., Creech J.L., Whelan J.G., Johnson M.N. Liver damage and angiosarcoma in vinyl chloride workers. A systematic detection program. J Am Med Assoc. 1974;230:64–68. [PubMed] [Google Scholar]
- 4.Almogy G., Lieberman S., Gips M., et al. Clinical outcomes of surgical resections for primary liver sarcoma in adults: results from a single centre. Eur J Surg Oncol. 2004;30:421–427. doi: 10.1016/j.ejso.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Holden C.A., Spittle M.F., Jones E.W. Angiosarcoma of the face and scalp, prognosis and treatment. Cancer. 1987;59:1046–1057. doi: 10.1002/1097-0142(19870301)59:5<1046::aid-cncr2820590533>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 6.Maluf D., Cotterell A., Clark B., Stravitz T., Kauffman H.M., Fisher R.A. Hepatic angiosarcoma and liver transplantation: case report and literature review. Transpl Proc. 2005 Jun;37(5):2195–2199. doi: 10.1016/j.transproceed.2005.03.060. PMID: 15964377. [DOI] [PubMed] [Google Scholar]
- 7.Young R.J., Brown N.J., Reed M.W., Hughes D., Woll P.J. Angiosarcoma. Lancet Oncol. 2010;11:983–991. doi: 10.1016/S1470-2045(10)70023-1. View Article : Google Scholar : PubMed/NCBI. [DOI] [PubMed] [Google Scholar]
- 8.Kumar A., Sharma B., Samant H. StatPearls. StatPearls Publishing; Treasure Island (FL): 2021 Jan. Liver angiosarcoma. [Updated 2020 Oct 15] [Google Scholar]
- 9.Chien CY, Hwang CC, Yeh CN, et al: Liver angiosarcoma, a rare liver malignancy, presented with intraabdominal bleeding due to rupture - a case report. World J Surg Oncol. 10:232012. [DOI] [PMC free article] [PubMed]
- 10.Tsai C.C., Hsieh J.F., Han S.J., Mo L.R. Hemoperitoneum secondary to biopsy of the hepatic angiosarcoma. Chin J Radiol. 1999;24:37–40. [Google Scholar]
- 11.Lee S.W., Song C.Y., Gi Y.H., et al. Hepatic angiosarcoma manifested as recurrent hemoperitoneum. World J Gastroenterol. 2008;14:2935–2938. doi: 10.3748/wjg.14.2935. [DOI] [PMC free article] [PubMed] [Google Scholar]