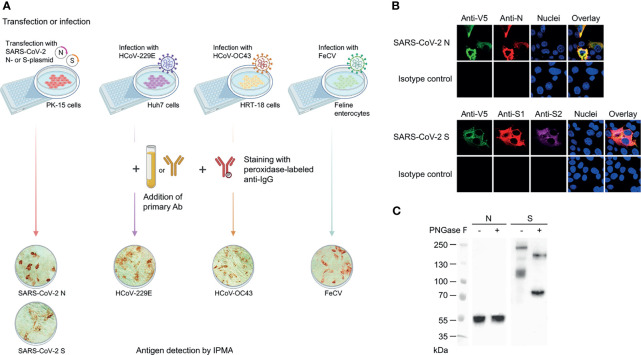
Figure 1.
IPMA assay for determination of antibody titers against SARS-CoV-2 S or N, or HCoV-229E, HCoV-OC43 and FeCV. (A) Assay principle and representative images of positive IPMA staining. For SARS-CoV-2, we used PK-15 cells transfected with an expression plasmid for V5-tagged S- or N-protein. For HCoV-229E, HCoV-OC43 and FeCV, we used virus-infected Huh7 cells, HRT-18 cells and feline enterocytes, respectively. Either serum, plasma, pleural fluid, ascites or purified antibody were used as primary antibodies; peroxidase-labelled goat anti-human or rabbit anti-cat IgG were used as secondary antibodies. (B) Staining with commercial antibodies confirmed SARS-CoV-2 N and S expression in transfected PK-15 cells, as evident from strong colocalization of anti-V5 and anti-N staining (top panels); and anti-V5, anti-S1 and anti-S2 staining (bottom panels). (C) Western blot showing proper expression of N and S in the transfected PK-15 cells, with full-length S undergoing S1/S2 cleavage and N-glycosylation, as evident from PGNase F treatment on the cell lysates.